Memorization Tips and Tricks
Key Questions
-
You have to go step by step, from easier ones to complex ones.
You have to learn a lot about alkanes, alkenes, alkynes. Then you have to practice how to draw them or how to give them IUPAC name. Then start with cycloalkanes, cycloalkenes, ...
Eventually start with complex ones: alcohols, phenoles, ethers...
Here is a short list of most important functional groups.
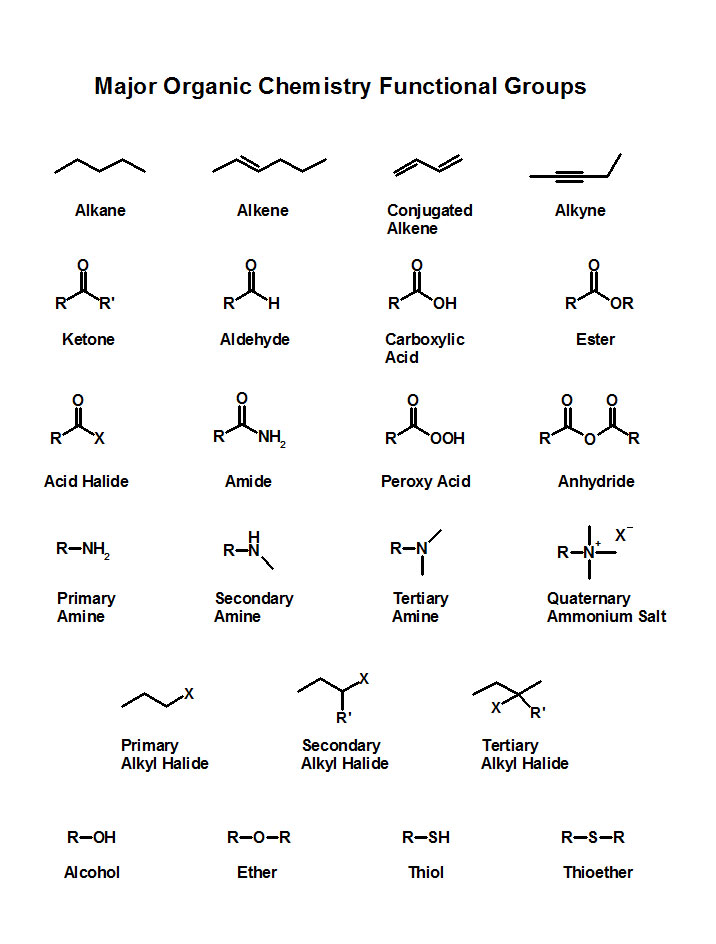
-
You mean how you can spot one on a compound? It's merely the part of the compound that branches away from the main chain.
Let's say you had this, N,N-dimethylacetoacetamide:
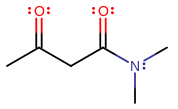
You could say that the main chain is the carbon attached directly to the nitrogen from the left (carbon-1) all the way to the far left carbon (carbon-4).
Then, the carbonyl groups would be the
#"C"="O"# of carbon-1 and carbon-3. The only other functional group is the secondary amine group on carbon-1. You can tell it's secondary from my writing N,N, which implies two substituents on the nitrogen. You could also say that the carbonyl on carbon-1 along with the#"N"("CH"_3)_2# can be coupled together as one functional group called an amide.If you want to be able to remember these, try quizzing yourself with flash cards.