Lewis Dot Diagram
Key Questions
-
Answer:
How else but by counting up (i) the number of valence electrons on the neutral atoms, and (ii) the charge on the atom or ion............?
Explanation:
Now we build Lewis structures by elaborating from neutral atoms, and of course, we have to account for the charge on the atom or radical ion.
Take for example
#"nitrate ion"# ,#NO_3^(-)# . Nitrogen, Group V, has 5 valence electrons; oxygen, Group VI, has 6 valence electrons. And we throw in another electron, so that we have#5+3xx6+1=24# #"valence electrons"# , i.e.#"12 electron pairs"# in the Lewis structure of#NO_3^-# to distribute around 4 centres.And thus we get
#O=N^+(-O^-)_2# . From the left, around the doubly bound oxygen there are#2+2# lone pair electrons on oxygen, (i)#4# electrons, and in the#O=N# bond, (ii)#4# electrons, NO lone pairs on the cationic, quaternized nitrogen, and (iii)#2xx8=16# electrons on the formally singly bound oxygens, each of which bears a NEGATIVE charge: thus 24 valence electrons as required for the Lewis structure.So all we do is to take the number of valence electrons (which is given by the atom's Group number), and add these numbers together, and add or subtract depending on the negative or positive charge of the species.
Can you do the same for
#PO_4^(-3)# ? There must be 32 electrons to distribute, i.e. 16 electron pairs. -
In a Lewis structure, atoms that are bonded covalently are represented by a single line joining the two atoms, which are represented by the element's chemical symbol. Covalent bonds occur mainly in diatomic molecules, such as hydrogen, nitrogen, fluorine, chlorine, bromine, iodine, and astatine.
The Lewis dot diagram for the covalent bonding of chlorine, (
#Cl_2# ), would be: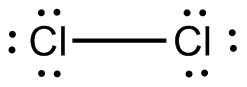
When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is:
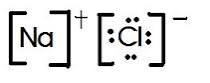
-
Answer:
Well, it is a diagram that represents the distribution of VALENCE electrons around atoms in a molecule...
Explanation:
The modern chemical bond is conceived to be region of high electron density between two positively charged atomic nuclei such that internuclear repulsion is negated and an attractive force results between the positively charged nuclei and the intervening electron cloud. The electrons surround individual atoms (and atoms bound in molecules) in orbitals whose shapes correspond to the Platonic solids, in such a way that the electron pairs are mutually repel each other....
And so it is important to account for the valence electronic configuration of an atom, as an atom, or as an atom in a molecule. And thus Lewis dot treatment distributes the valence electrons. And we can easily find the number of valence electrons for a given atom by noting its Group number in the Periodic Table, which number gives required the number of electrons.
For a simple example, consider ammonia,
#NH_3# , the which has 5 nitrogen valence electrons, and 3 electrons from the hydrogens...we distribute these valence electrons around the molecule...and the number of BONDING and NON-BONDING electron pairs DIRECTLY influences molecular geometry.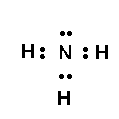
Nothing I say here will replace reading the relevant chapters in your text, so get to it...and we will be available for any questions that arise.