Question #a9c4a
1 Answer
The minimum energy required for isomerization is 267 000 J/mol or
Explanation:
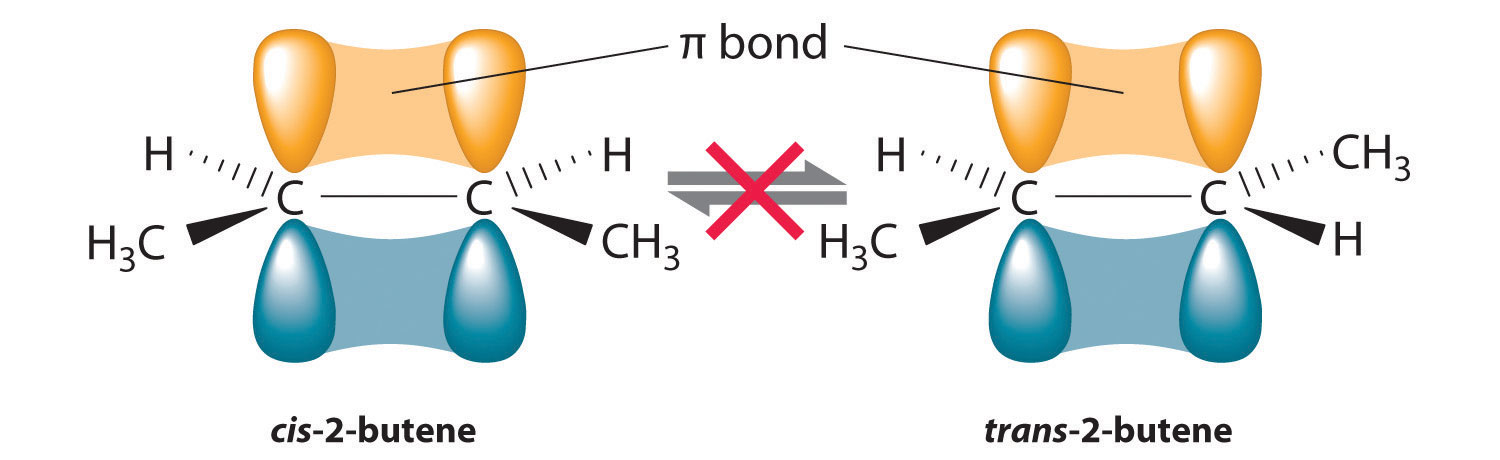
The isomerization of cis-but-2-ene to trans-but-2-ene requires breaking of the π bond.
The bond energy of a C-C σ bond is 347 kJ/mol.
The bond energy of a C=C double bond (σ + π) is 614 kJ/mol.
So the bond energy of a π bond is (614 – 347) kJ/mol = 267 kJ/mol =
267 000 J/mol.
On a molecular level, the bond energy of a π bond is
A quantum of light can excite a π electron to a π* orbital and allow free rotation about the central C-C bond.

We can calculate the minimum frequency required.

