Does ammonia have filled #pi# orbitals if it doesn't have any #pi# bonds?
1 Answer
Oct 18, 2017
Well, look up the MO diagram of
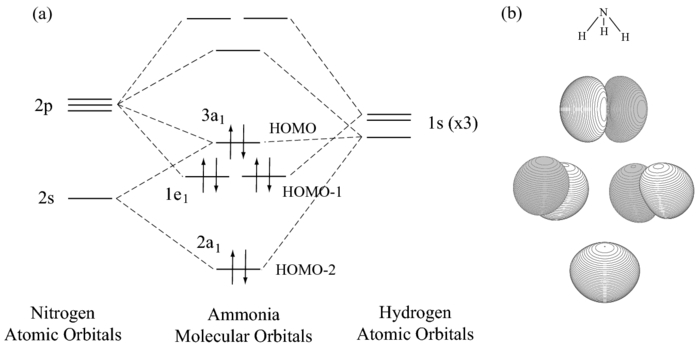
So yes, it has filled
Note that they aren't really making
The HOMO-2 and HOMO-1 orbitals are what were involved in making the three

