Does an electron move from one allowed orbit to another only when it absorbs or emits a fixed amount of energy?
1 Answer
Aug 18, 2017
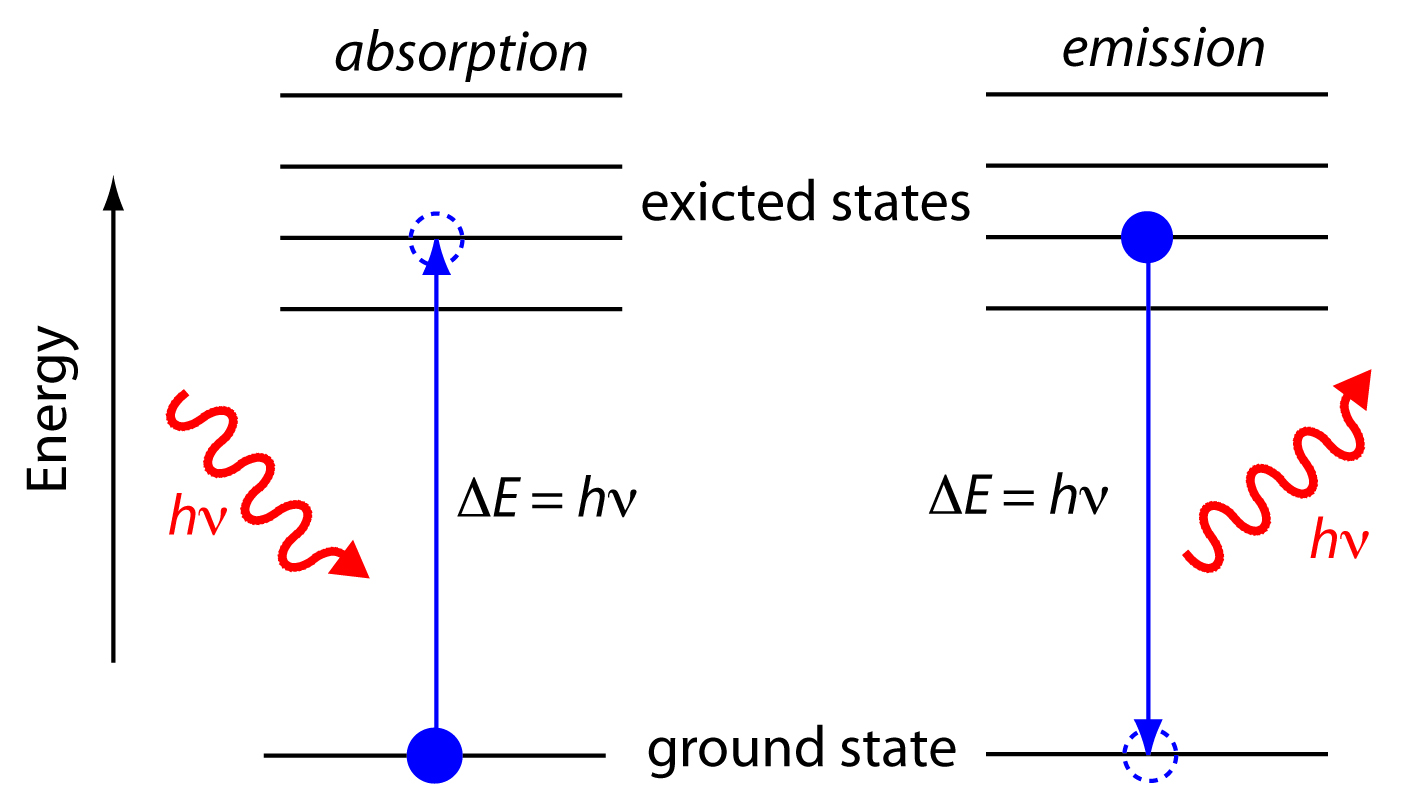
Yes, that is what is meant by quantization of energy. It is not to say that the electron is actually in a fixed orbit (it is a simplified model), but there is a discrete set of energy level gaps in any given atom.
A given quantum of energy,
- Energy input of
#hnu# describes the energy given by one absorbed photon that causes excitation of an electron to a higher energy level, based on the frequency#nu# . - Energy output of
#hnu# describes the energy in one emitted photon that was due to relaxation of an electron from a lower energy level, based on the frequency#nu# .
And if the electron being excited were to receive less than enough energy to get to the next energy level, it doesn't get there and falls back down to where it started.

