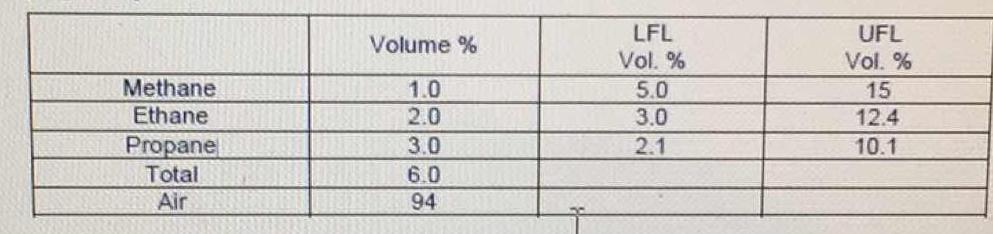
Gas mixture at ambient pressure is composed of 1.0% methane, 2.0% ethane, and 3.0% propane by volume. What is the mole fraction of each component in the mixture (on combustible basis)? What is the lower flammability limit & the upper flammability limit?
1 Answer
May 26, 2017
The mole % for each is the same as the volume % given. The Flammability limits are also already given in the table.
Explanation:
For gases, relatively dilute at 'normal' pressures, the Ideal Gas Laws are a good approximation. In essence, a mole of any gas will take up the same volume as any other mole of gas. Therefore, the volume percentages are the same as the mole percentages.
The lower flammable limit is the minimum amount of a gas that is necessary to sustain a combustion. The upper flammable limit is the maximum amount of gas that could be ignited. More gas than this value will not ignite.
Of the three gases in this mixture, only propane is in the "flammable zone".

