How can I convert the 3,3-dimethylhexane #C_3−C_4# bond from bond line notation to a Newman projection?
1 Answer
Jan 23, 2015
Step 1. Draw the structure of 3,3-dimethyl hexane.
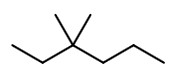
Step 2. Convert to a wedge-dash structure at C-3 and C-4.
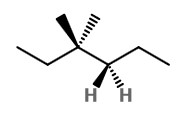
Step 3. Identify the groups on C-3 and C-4.
The groups on C-3 are CH₃, CH₃, and CH₂CH₃. Those on C-4 are H, H, and CH₂CH₃.
Step 4. Draw a template for a Newman projection.
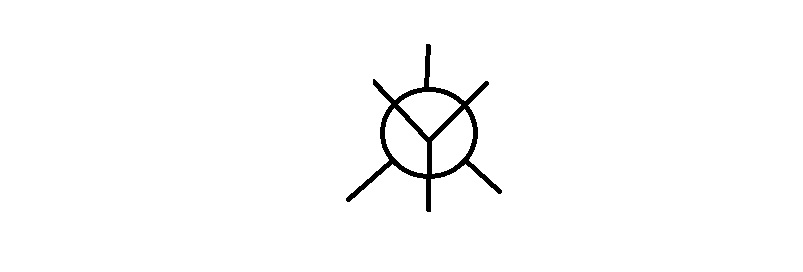
Step 4. Attach the groups to the carbons of your template.
View the molecule from the upper left.
The groups on C-3 go on the front carbon atom. The bulky CH₂CH₃ group goes on the bottom, and the CH₃ groups go on the other bonds.
The groups on C-4 go on the back carbon. The bulky CH₂CH₃ group on the top, and the H atoms go on the other bonds.
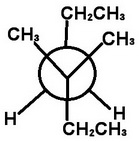
This is the most stable conformer. It has the bulky ethyl groups anti to each other.

