How can I write the formula for calcium nitride?
1 Answer
Explanation:
The correct answer is
Let us see how we got the answer; Look at the electronic arrangement of Ca and N atom.
Ca ( Z= 20) has 20 electrons with following electronic configuration. 1
It loses two electron in its 4s subshell to achieve stability and forms ion
N ( Z=7) on the other hand has seven electrons and wants to gain three electrons to achieve stable noble gas configuration.Nitrogen atom on gaining three electrons forms negative nitride ion,
N ( Z=7) = 1
Two Nitrogen atoms gains three electrons each ( total of six) from three Ca atoms, each Ca atom loses two electrons (total of six) to two Nitrogen atom , in this process each Ca atom becomes
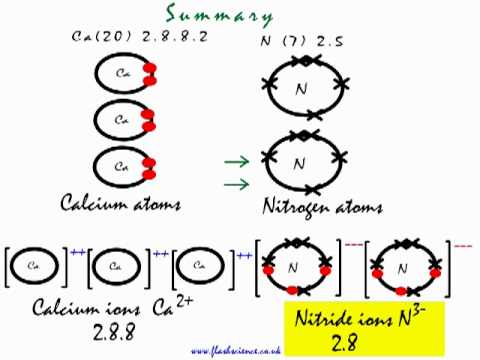
so in all we have three
so the formula becomes


