How do you draw all the stereoisomers of 1-bromo-2-chlorocyclohexane?
1 Answer
Jan 14, 2016
You draw all possible stereoisomers and then delete any meso compounds.
Explanation:
Step 1. Draw the bond-line structure.
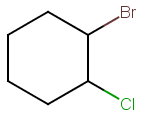
Step 2. Identify the chiral carbons.
Step 3. Calculate the maximum number of stereoisomers.
There are
Step 4. Draw all the possibilities.
Draw one structure with the bonds UpUp and its mirror image.
Then draw another structure with the bonds UpDown and its mirror image.
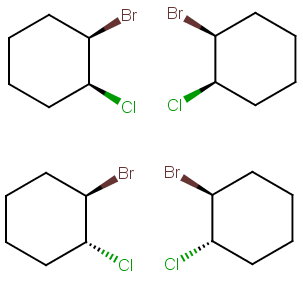
There are no internal mirror planes, so there are no meso compounds.
There are four stereoisomers.
The top structures are a pair of enantiomers, and the bottom structures are a pair of enantiomers.

