How does carbon 12 change to carbon 14?
1 Answer
May 17, 2016
It doesn't
Explanation:
C-12 contains 6 protons and 6 neutrons and is a stable isotope of carbon.
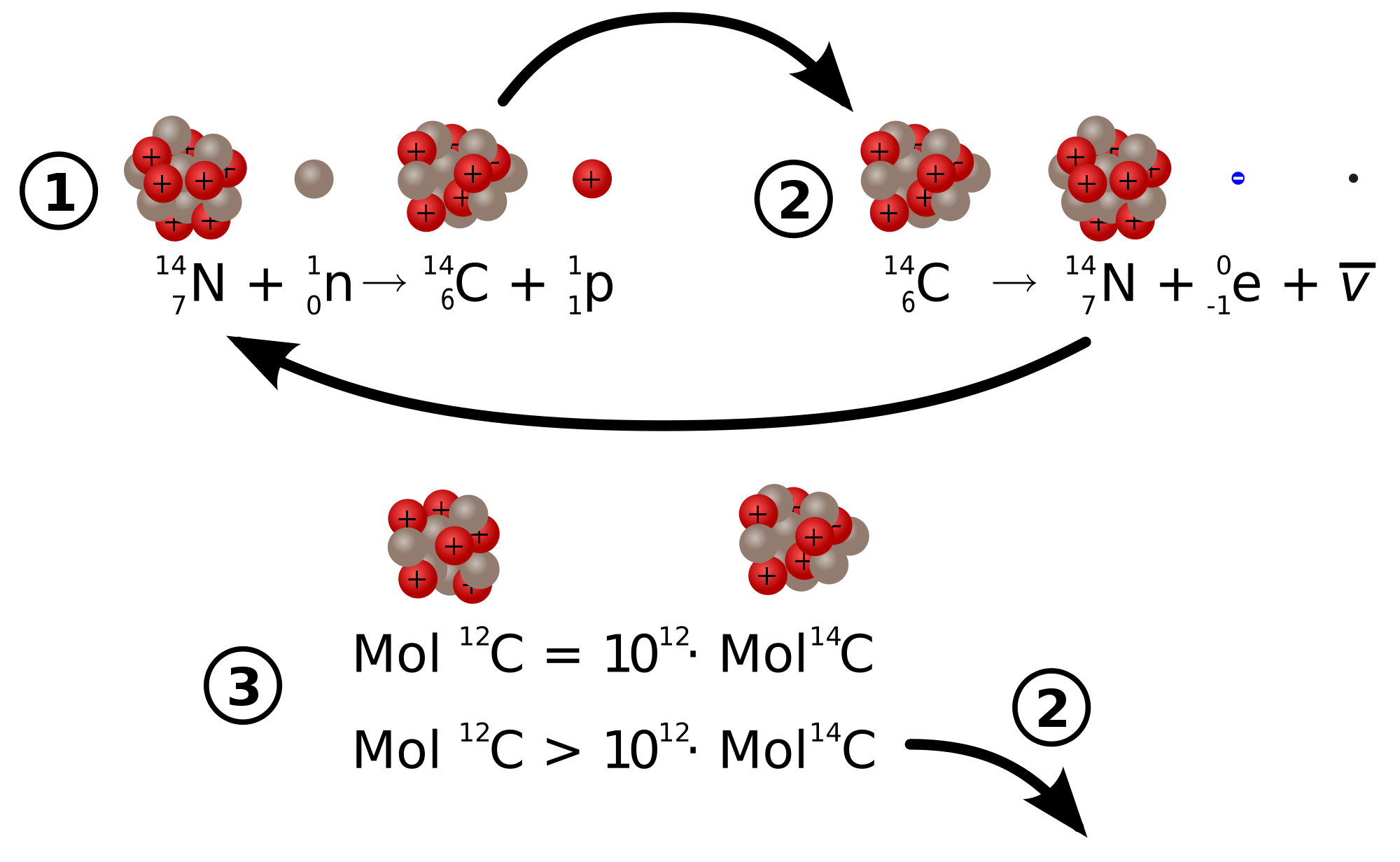
C-14 atoms have 6 protons and 8 neutrons.
C-14 forms in the upper atmosphere when cosmic rays enter the upper atmosphere, forming neutrons that collide with N-14 atoms and covert them to C-14 .
This video explains how to determine the number of subatomic particles in the two isotopes of carbon.
Carbon-12 atoms have stable nuclei because of the 1:1 ratio of protons and neutrons.
Carbon-14 atoms have nuclei which are unstable. C-14 atoms will undergo alpha decay and produce atoms of N-14. Carbon-14 dating can be used to determine the age of artifacts which are not more than 50,000 years old.
Hope this helps!

