How does radiation change across the electromagnetic spectrum?
1 Answer
Three things change: frequency, wavelength and method of production.
Explanation:
The electromagnetic spectrum is a family of waves that have the same essential nature (they are vibrations in electric and magnetic fields) but different wavelength/frequency and in some cases a different method of production.
The spectrum is often represented by a continuous sinusoidal wave that has a shortening wavelength toward one end – see the diagram below for this.
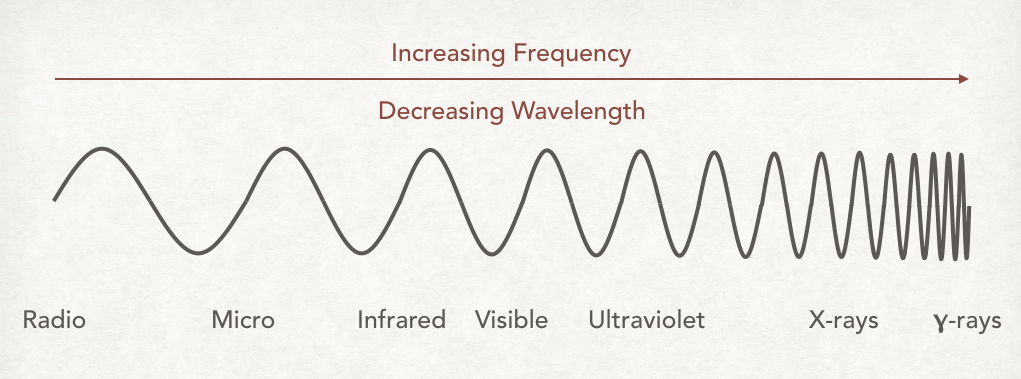
Note speed is the same for all electromagnetic waves and as frequency increases the wavelength decreases.
As you can see radio waves have the longest wavelengths and gamma-rays have the shortest. The spectrum is divided into seven major regions according to wavelength: radio, microwaves, infrared, visible light, ultraviolet, x-rays and γ-rays.
Methods of production
The waves in some of the regions are produced differently to other regions. For example whereas microwaves are produced by oscillating electrons in wires/aerials visible light is produced when electrons in atoms drop down energy levels. Here is a table with rough wavelength ranges for the regions with their methods of production.
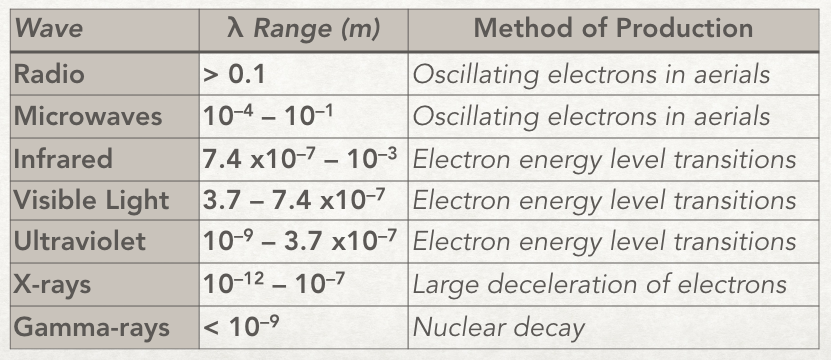
The method of production is an important distinction where there is an overlap of wavelength. For example the x-ray and γ-ray regions overlap in wavelength; some x-rays have the same wavelength as some γ-rays. But we still call x-rays x-rays and not γ-rays because they were produced by a linear accelerator and not by nuclear decay.
Another thing to be aware of:
Frequency is related to wave energy, so high frequency γ-rays have a higher energy than all other electromagnetic waves. The frequency of waves in the UV part of the spectrum and above means they have enough energy to remove electrons from stable atoms. That makes UV, x-rays and γ-rays ionising radiation.

