Knowing the following, what caused some of the positive particles to be deflected in this experiment?
In 1911, Ernest Rutherford carried out an experiment to confirm the structure of the atom. In this experiment, he fired positive particles at a very thin layer of gold foil. Most of the particles passed straight through but a small number of the positively charged particles were deflected. path of positive particles gold atoms.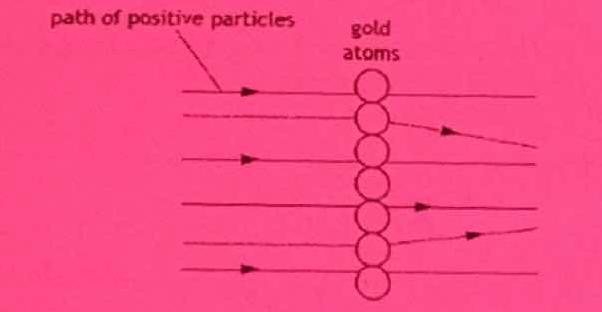
In 1911, Ernest Rutherford carried out an experiment to confirm the structure of the atom. In this experiment, he fired positive particles at a very thin layer of gold foil. Most of the particles passed straight through but a small number of the positively charged particles were deflected. path of positive particles gold atoms.
1 Answer
Explanation:
This experiment is usually poorly understood, because we do not appreciate the infinitesimal thinness of the gold foil, the gold film, that Rutherford utilized; it was only a FEW atoms thick.
The deflection of the
Capisce?

