What alkane of molecular C8H18 gives a single product of monohalogenation when heated with Cl2? Write the IUPAC name for the product of monohalogenation?
1 Answer
Aug 25, 2015
The alkane is 2,2,3,3-tetramethylbutane.
Explanation:
Start with an ethane molecule,
You need six more carbon atoms, so replace each of the six
You get
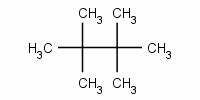
Its IUPAC name is 2,2,3,3-tetramethylbutane.
The equation for chlorination is

