What are aprotic solvents?
1 Answer
Jan 5, 2015
An aprotic solvent is a solvent that has no O-H or N-H bonds.
The "a" means "without", and "protic" refers to protons or hydrogen atoms.
The specific meaning of aprotic is that the molecules have no H atoms on O or N.
This means that the molecules cannot form H-bonds with themselves, but they may accept H-bonds from other molecules.
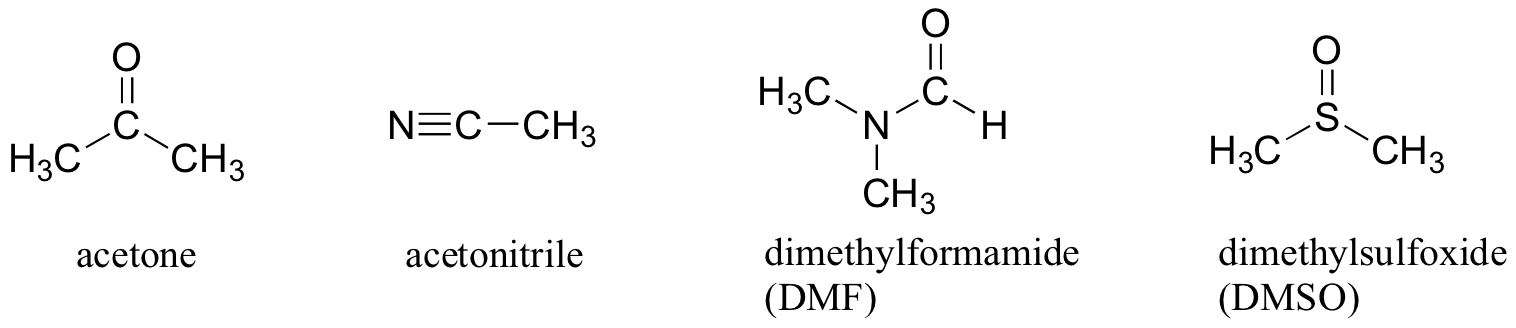
For example, acetone does not have an O-H group, but it has a C=O group that can participate in H-bonding.
So acetone is an aprotic solvent.
Some other common aprotic solvents are listed below.