What are Reducing Agents? And why are they used in chemical reactions?
1 Answer
Jun 16, 2018
see below
Explanation:
Chemical reducing agents are substances, neutral molecules or ions, that can easily oxidate themselves to a bigger oxidation number, reducing another substance.
they generally have a low Oxidation number (as
Other usefull reducing agents are: some metals as Mg, Al, Zn and
Their force is tabelled in the standard reduction potentials (see figure) where they are in the right on the bottom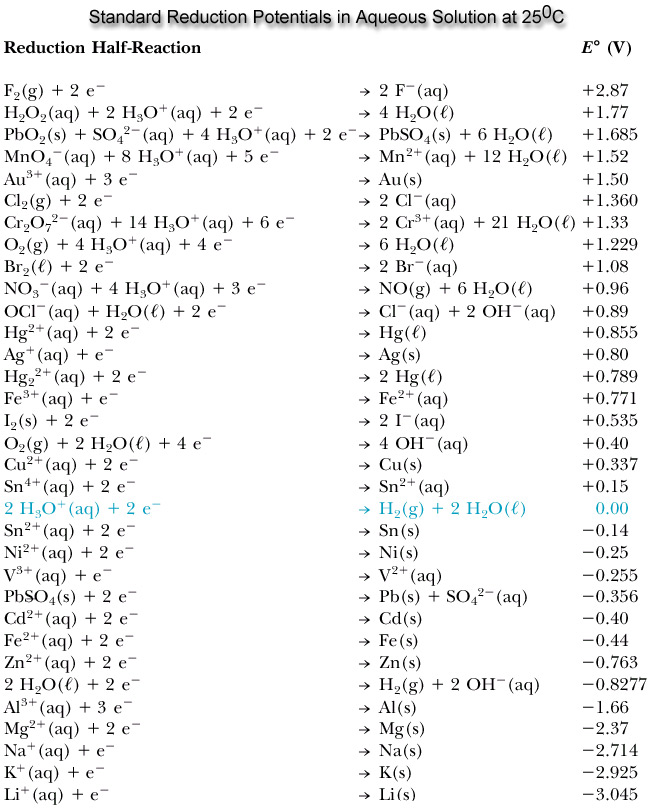
they are used to reduce oxydant substances, and they are usen in alimentary industries for prevention toware oxidation of foods and to conservation of food
