What are the chiral centers of the molecule of nicotine?
1 Answer
Nov 24, 2015
There's only one, and it's S.
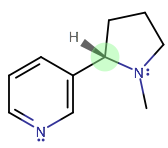
The highest to lowest priority is (1,2,3,4):
- tertiary amine (highest atomic number here;
#-"NC"_2# ) - aromatic
#sp^2# (taken similar to tert-butyl, i.e.#-"CC"_3# ) - methylene (
#-"CH"_2# ) - hydride (
#-"H"# )
You get that from going clockwise and crossing the hydride, but since the hydride is facing forwards rather than backwards, the configuration is the reverse of R, which is S.

