What does salt do to ice?
1 Answer
Salt, when placed onto ice, actually slightly lowers the freezing point of ice so that it melts more easily. That's why icy roads were once salted to make them safer.
It's called the colligative properties of a solute.
When you place a solute (like salt) onto a solvent (like ice), it makes the ice less pure. It decreases its chemical potential
From the following equation, we can rationalize this:
#\mathbf(mu_j = mu_j^"*" + RTlnchi_j)# where
#mu_j^"*"# is the chemical potential of the pure solvent and#chi_j = (n_j)/(n_i + n_j)# is the mol fraction of the solvent,#n_i# is the#"mol"# s of solute, and#n_j# is the#"mol"# s of solvent.
The resultant chemical potential must decrease if
Notice that
That means
Finally, we can see what this means by looking at this graph:
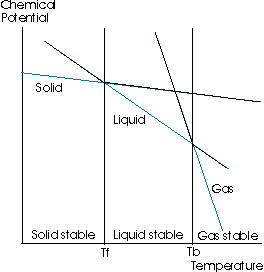
where the chemical potential increases upwards on the y-axis, and the temperature increases rightwards on the x-axis.
When the chemical potential decreases, what actually happens in this graph is that the diagonal straight line belonging to the liquid phase moves downwards.
(black line towards the yellow line)
That means the intersection between the liquid line and the solid line moves to the left.
Therefore, the freezing point

