Which factors promote the formation of solutions?
1 Answer
The major factor affecting solubility is intermolecular forces.
To form a solution we must:
1. Separate the particles of the solvent.
2. Separate the particles of the solute.
3. Mix the particles of solvent and solute.
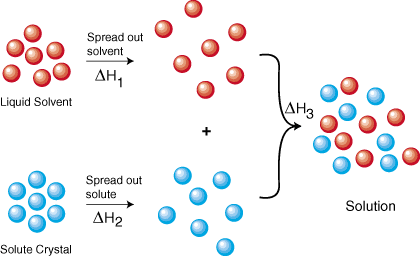 img.sparknotes.com
img.sparknotes.com
For the solution process to be favourable,
If both solvent and solute are nonpolar, all the
If both solvent and solute are polar, all the
LIKE DISSOLVES LIKE.
If a nonpolar solute such as oil mixes with a polar solvent like water,


