What happens on the cathode during the electrolysis of a copper(II) sulfate solution?
1 Answer
I assume you are referring to the electrolysis of copper (II) sulfate with copper electrodes. During the electrolysis of copper (II) sulfate, or
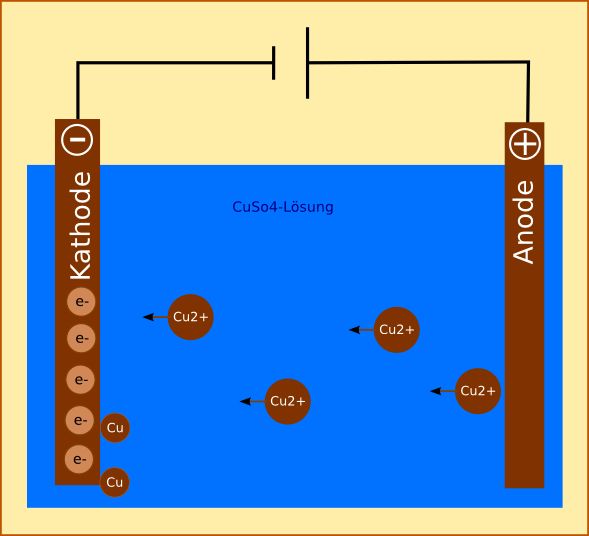
It is very important to make a distinction between the labelling of the cathode in electrolysis and in galvanic cells, as most of the time this causes a bit of confusion. In electrolysis, the cathode is the negative electrode, while in galvanic cells it acts as the positive electrode.
This difference is determined by the fact that in electrolysis, an electric current is used to drive a chemical reaction; however, in a galvanic cell, the opposite is true - a chemical reaction is used to produce an electric current.
So, this is what happens during the electrolysis of
When a direct current (DC) is applied, the cations will be attracted by the cathode and the anions will be attracted by the anode. Here's what takes place at the cathode:
Here's what takes place at the anode:
In time, this will ensure that the anode will dissolve, and that the cathode will increase in size. The concentration of the copper (II) sulfate solution will remain unchanged during electrolysis.
Here's a great video on the electrolysis of copper (II) sulfate:

