What is adiabatic calorimetry?
1 Answer
Adiabatic (from Greek a- "not", –dia- "through, batos "passable") calorimetry is calorimetry in which no heat is exchanged with the surroundings.
Explanation:
A runaway reaction occurs when it produces heat faster than it loses heat.
The worst case is when the reaction occurs adiabatically, that is, without any heat exchange with its surroundings.
Any heat causes the temperature to rise and speeds up the reaction, producing even more heat.
An adiabatic calorimeter is designed to study these run-away reactions.
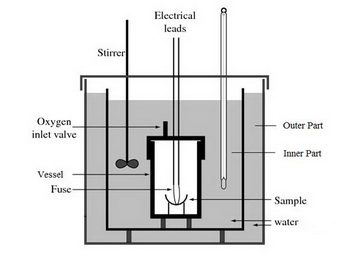
(from www.tawadascientific.com)
It is like a bomb calorimeter, except that the temperature in the outer part is controlled to follow the temperature of the inner part throughout the experiment.
Also, the room is usually air conditioned to a constant temperature to keep any errors as small as possible.

