What is the molecular geometry of CCl4? Draw its VSEPR and Lewis structure.
1 Answer
Explanation:
Lewis Structure
Here are the steps that I follow when drawing a Lewis structure.
1. Decide which atom is the central atom in the structure.
That will be the least electronegative atom (
2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom — a
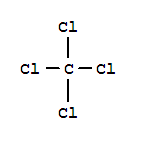
3. Draw a trial structure by putting electron pairs around every atom until each gets an octet. (Every atom in the trial structure has an octet.)
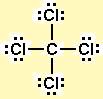
4. Count the valence electrons in your trial structure (32).
5. Count the valence electrons you actually have available.
6. The trial structure has exactly the same number of electrons as we have available.
Thus, the trial structure is the correct Lewis structure.
VSEPR Geometry
The Lewis structure shows that there are four electron regions about the central carbon atom.
The VSEPR model states that the electron regions around an atom spread out to make each region is as far from the others as possible.
For these clouds in
The bonds will emanate from the central atom at angles of 109.5 ° to each other.
The structure will be tetrahedral.



