What is the most stable conformer for 3-methylhexane, viewed along the #C_3-C_4# bond using Newman projections?
1 Answer
The most stable conformer has the ethyl groups anti to each other with a methyl-ethyl gauche interaction.
Step 1. Draw the structure of 3-methyl hexane.

Step 2. Identify the groups on C-3 and C-4.
The groups on C-3 are H, CH₃, and CH₂CH₃. Those on C-4 are H, H, and CH₂CH₃.
Step 3. Draw a template for a Newman projection.
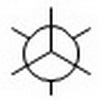
Step 4. Attach the groups in any order to the correct carbons of your template.
The groups on C-3 go on the front carbon atom. The groups on C-4 go on the back carbon.
Step 5. Generate the other two projections.
Rotate the front carbon of the first structure in 120 ° steps. You should get something like the three structures below (or their mirror images):

Step 6. Identify the most stable conformer.
The most stable conformer has the fewest gauche interactions.
The middle structure is the least stable, because it has two gauche interactions.
The other structures have only one gauche interaction. But the ethyl-ethyl repulsion should be greater than the methyl-ethyl repulsion.
The first structure is the most stable conformer.

