When Iodine (I2) is mixed with water, the water dissolves some iodine by forming a temporary charge. Does the oxygen from water bond with the Iodine or does the hydrogen?
1 Answer
The oxygen from water bonds with the iodine.
Explanation:
Pure iodine has a violet colour.
When iodine is in a nonpolar solvent , it stays violet.
Iodine and water can have dipole-induced dipole interactions, in which either end of the water dipole can induce a temporary dipole in the iodine molecule.
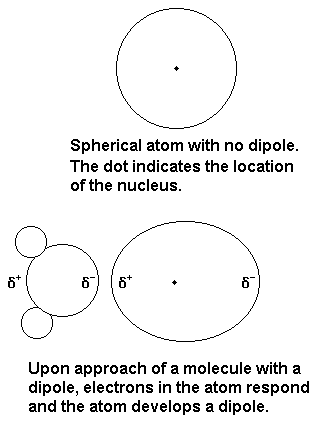
However, iodine can also act as a Lewis acid (an electron acceptor).
The interaction with the oxygen end of water becomes more important, because the lone pair electrons on water make it a Lewis base.
The molecules can form a loosely bound Lewis-type charge transfer complex, in which there is a partial transfer of electrons from the water to the iodine.
The formation of the complex changes the colour of light absorbed.
A solution of iodine in water is yellow-brown instead of violet.

The image above shows a solution of iodine in hexane on top and of iodine in water below.

