Which is more nucleophilic: the iodide ion (I−) or the fluoride ion (F−)?
1 Answer
I⁻ is a better nucleophile than F⁻ in polar protic solvents.
F⁻ is a better nucleophile than Br⁻ in polar aprotic solvents.
A protic solvent has an H atom bound to O or N. It can use its H atom to participate in H-bonding with a nucleophile.
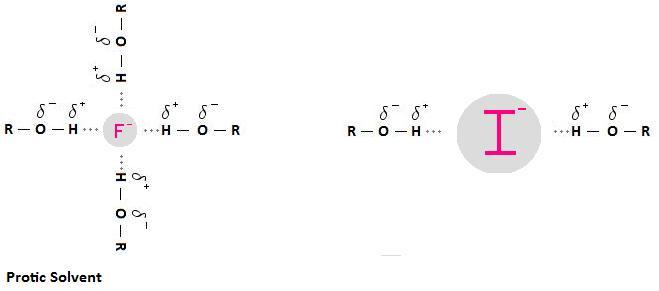
This creates a "shell" of solvent molecules around the nucleophile.
The nucleophile has to push this shell of solvent molecules out of the way to attack the carbon bearing the leaving group.
F⁻ is a small ion with a high charge density. It is tightly solvated.
I⁻ is a large ion with a low charge density. It is loosely solvated. There are only a few solvent molecules to push out of the way.
In polar protic solvents, the order of nucleophilicity is
I⁻ > Br⁻ > Cl⁻ > F⁻
A polar aprotic solvent does not have a hydrogen atom that can participate in hydrogen bonding.
Some typical polar aprotic solvents are
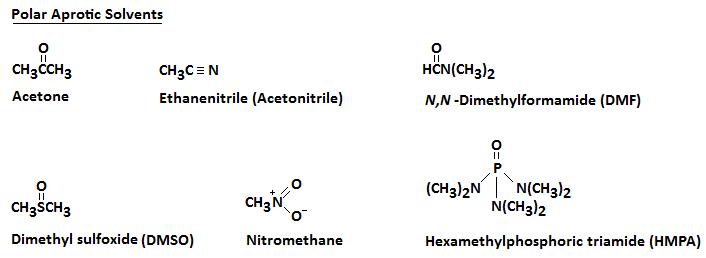
In all of them, the negative ends of the dipoles point away from the molecule. It is easy for them to solvate cations.
The positive ends of the dipoles are closer to the middle of the molecule. It is difficult for them to get close to the anions.
The result is that the nucleophile has few molecules in its solvent shell. The nucleophile can more easily attack the substrate.
F⁻ becomes a much better nucleophile.
In polar aprotic solvents, the order of nucleophilicity is reversed:
F⁻ > Cl⁻ > Br⁻ > I⁻

