Which is predicted to have the shorter sulfur–oxygen bonds, #SO_3# or #SO_3^(2-)# ?
1 Answer
Aug 25, 2015
Sulfite ion has the shorter
Explanation:
The Lewis structure of
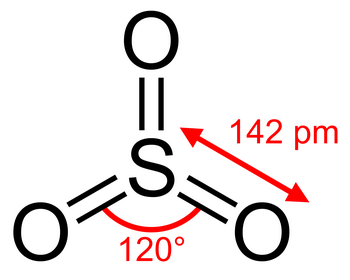
(from chemistry.stackexchange.com)
It has a total of six σ and π bonds to the three
The average bond order of an
The Lewis structure of
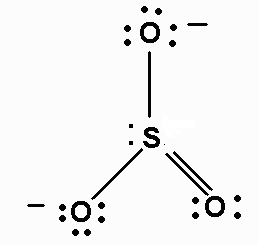
It is a resonance hybrid, with a total of four σ and π bonds to the three
The average bond order of an
Since bond length decreases as bond order increases,
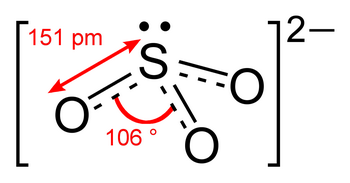
(from en.wikipedia.org)

