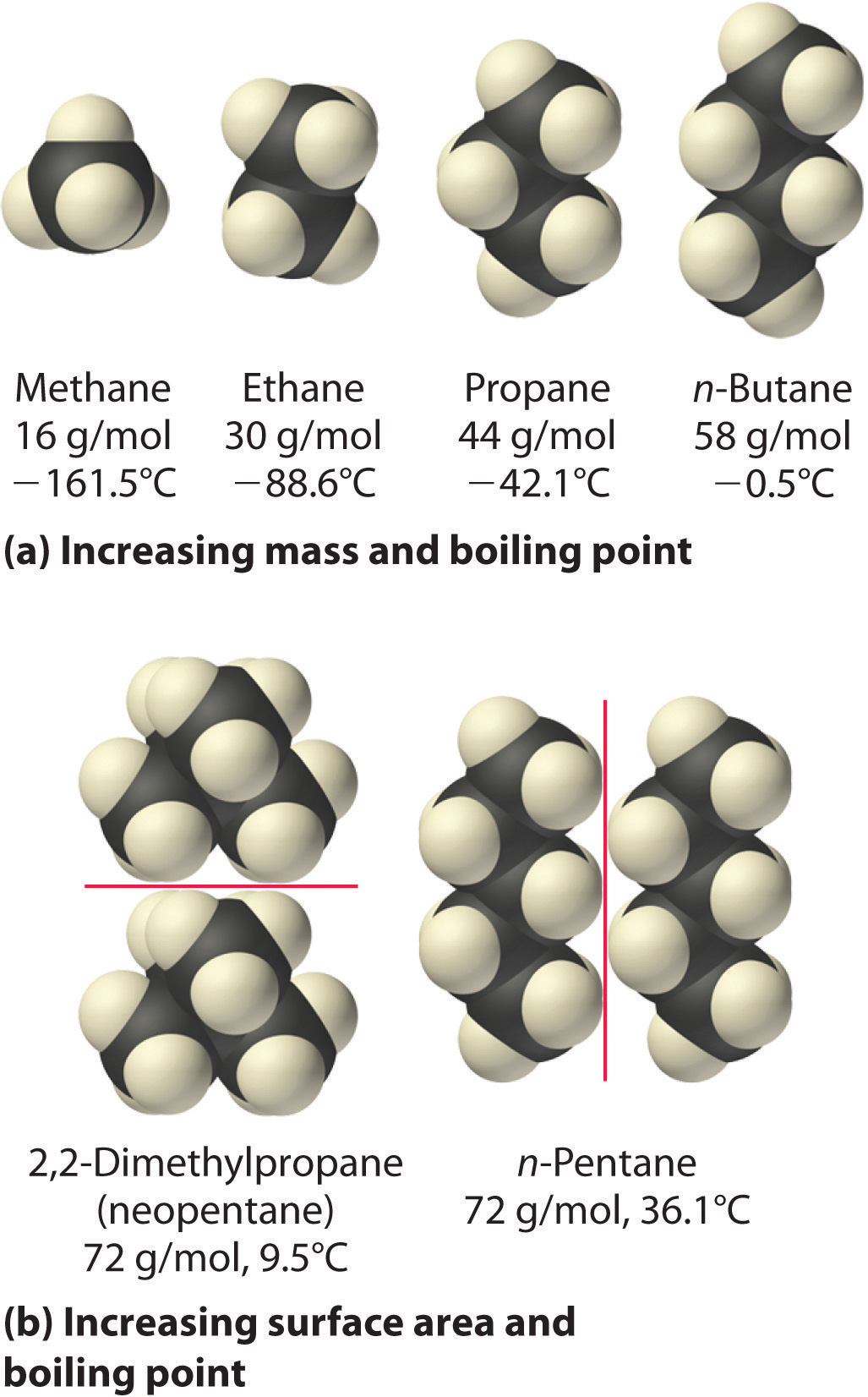
Which molecule would have the largest dispersion molecules forces between other identical? (A) CH4 (B) C3H8 (C) C2H6 (D) C2H4 (E) C4H10
1 Answer
The answer is E)
When judging the strength of intermolecular forces in compounds that only exhibit weak van der Waals interactions, or London dispersion forces (LDF), you have to go by two things
- Molar mass - the size of the molecule in question - in your case, the longer the carbon chain and the bigger the molar mass, the stronger the LDFs will be;
- Surface area - the shape of the molecule - the larger the surface area, the stronger the LDFs;
So, let's analyze the compounds you're dealing with. A measure of the strength of the LDFs will be the compounds' boiling point. The stronger the dispersion forces, the higher the boling point will be.
If you take into account the two rules I've mentioned, you'll end up, in order of increasing boiling point, i.e increasing strength of LDFs, with
An interesting thing takes place with butane, or
This is important to note because it offers an example on how surface area affects the strength of the LDFs. Because the branched-chain isomer has a smaller surface area, you could predict a lower boiling point when compared with straight-chain butane.
Indeed, isobutane has a boiling point of
Here's how the trend in hydrocarbon boiling point looks like