What is the role of buffer solution in complexometric titrations?
1 Answer
The buffer adjusts the pH to ensure that the reaction goes to completion.
Explanation:
A complexometric titration uses the formation of a coloured complex to indicate the endpoint.
EDTA, often written as
It has four carboxyl groups and two amine groups that can act as electron pair donors (Lewis bases).
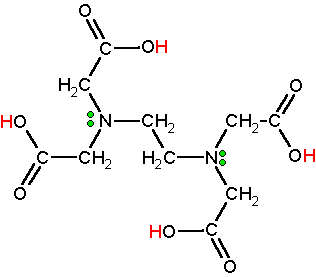 www.chm.bris.ac.uk
www.chm.bris.ac.uk
EDTA is often used as the disodium salt,
It reacts with many metal ions to form a complex:
The complex has the structure
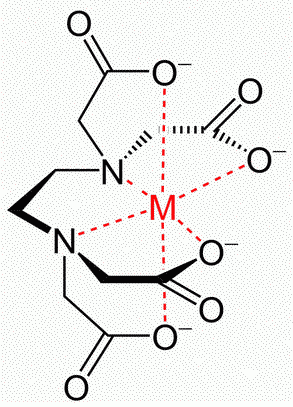 Metal-EDTA
Metal-EDTA
(From upload.wikimedia.org)
Carrying out the reaction in a basic buffer solution removes the
This moves the position of equilibrium to the right and favours formation of the complex (Le Châtelier's Principle).
Also, for EDTA,
Thus, if the solution is buffered to about pH 10.3, most of the EDTA will exist as
The metal ions will not have to remove the hydrogen ions from
They can react directly according to the equation
Above pH 10.3, most metal ions react quantitatively with EDTA.

