Write the structural formulae of the two isomers with the molecular formula #C_4H_8O# to illustrate functional group isomerism?
2 Answers
At first, just disregard the
Explanation:
Since the net formula of a
This can be done in only two different ways: at the end or somewhere in the middle.
Your isomers are (pictures from Wikipedia):
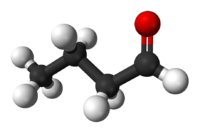
butanal or (butyric aldehyde)
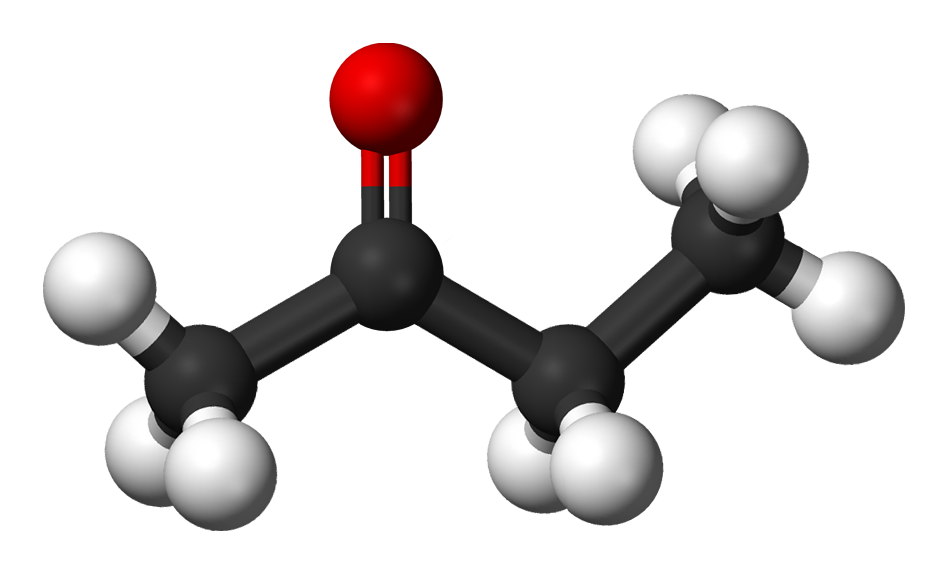
butanone (or methyl ethyl ketone)
The functional difference between aldehydes and ketones is that only the aldehyde can easily be oxidised to form a carbonic acid, in this case butanoic acid (or butyric acid). The ketones can only be destructively oxidised, by more powerful reactants.
An alternative way of assessing organic formulae is to invoke degrees of unsaturation. More isomers are possible.
Explanation:
As explained in the linky,
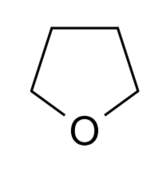
THF (so-called) is a solvent that is widely used in organic and organometallic chemistry. It is water soluble, and higher boiling than diethyl ether, and its structure illustrates the richness of organic chemistry: only a few atoms, but a wealth of possible structural formulae.


