Question #50944
1 Answer
Oct 11, 2014
The periodic trend of reactivity depends on whether the elements are metals or nonmetals.
For metals, reactivity increases down a group and from right to left across a period.
Nonmetals do the opposite. For nonmetals, reactivity increases up a group and from left to right across a period.
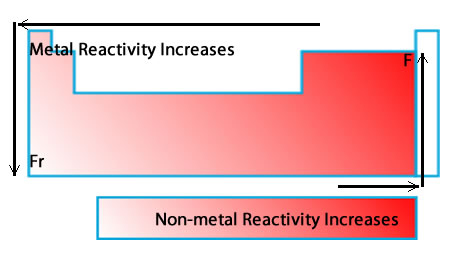
Francium is the most reactive metal, and fluorine is the most reactive nonmetal.

