Question #73027
1 Answer
In order to get an idea about how the atomic orbitals of the central atom, which in your case is phosphorus, will combine to form hybrid orbitals in the hexafluorophosphate ion,
The hexafluorophosphate ion will have a total of 48 valence electrons - 5 from phosphorus, 7 from each of the 6 fluorine atoms, and 1 from the negative charge carried.
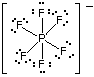
The phosphorus atom forms 6 covalent bonds with the 6 fluorine atoms, and it has no lone pairs present. This means that it'll have a steric number equal to 6.
The steric number will determine the hybridization of the central atom, i.e. the number of hybrid orbitals an atom has must be equal to its steric number.
In your case, phosphorus must have 6 hybrid orbitals .
Now take a look at phosphorus' electron configuration
Notice that its ground state configuration is (the diagram on the left)
In order to be able to form 6 bonds, an electron from the 3s-subshell will be promoted to an empty d-orbital. This will allow the excited-state phosphorus to form 5 bonds.
The electron that gives the ion its negative charge will occupy a second 3d-orbital. As a result, 6
- one 3s-orbital;
- three 3p-orbitals;
- two 3d-orbitals.


