Question #b3c21
1 Answer
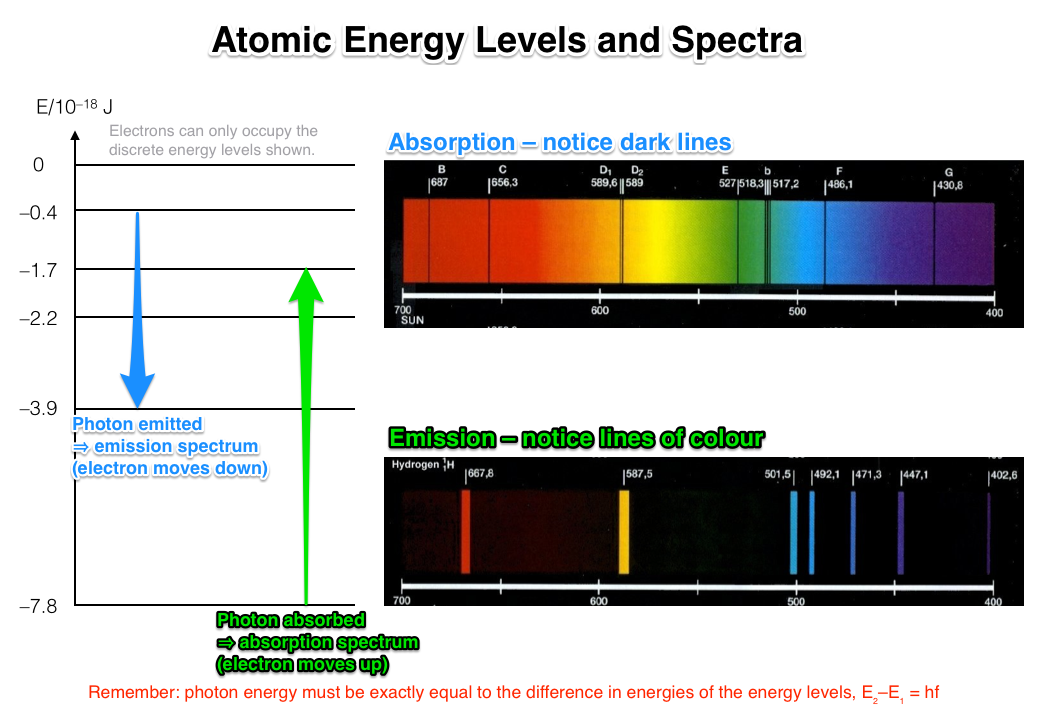
Emission: only some wavelengths present in the spectrum (a line spectrum ).
Absorption: a continuous spectrum with some wavelengths missing.
Explanation:
Orbital electrons in atoms can occupy only discrete energy levels. The atoms of each element have exactly the same discrete energy levels.
An emission spectrum is produced by the excitation of orbital electrons. Electrons move up energy levels during excitation (energy came from somewhere to excite the electrons). In order to conserve energy as they move down energy levels, energy is emitted in the form of electromagnetic radiation, i.e. visible light.
(Note that because discrete amounts of electromagnetic energy are emitted they are emitted in "particles" called photons .)
In this process only certain wavelengths of light are emitted, which gives rise to the emission spectrum containing only certain wavelengths of light. The wavelengths correspond to the energy difference between the levels that the electron dropped down.
Where E refers to the energy levels, h is Planck's constant, c is the speed of light and λ is the wavelength.
An absorption spectrum is produced by essentially the reverse process. Electrons can absorb energy from photons of light that interact with them. Thus they become excited and move to higher energy levels in the atom (to conserve energy). The photon energy must correspond exactly to the difference in energy levels that the electron moved between (same equation as above applies). Thus only specific wavelengths are absorbed from the passing light.
The wavelengths that are not absorbed continue on their merry way to the observer who produces a spectrum and notices that specific wavelengths are missing.
Here's a summary image/diagram that I hope helps to illustrate the above points.