How does a catalyst affect collision theory?
1 Answer
May 12, 2016
The catalyst does not affect the rate of collision since it does not change the kinetic energy (
Explanation:
A catalyst by definition is a substance which is not a reactant nor a product that increases the rate of the reaction by lowering its activation energy (see graph below).
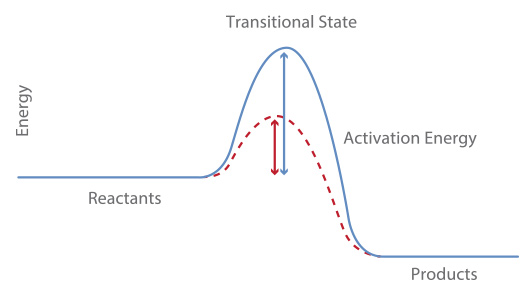
Note that the catalyst does not affect the rate of collision since it does not change the kinetic energy (
The kinetic energy depends only on temperature, and catalyst does not change the temperature of a reaction.
Chemical Kinetics | A Model for Chemical Kinetics & Catalysis.

