What volume of a 6.0 M #HCl# solution is required to make 250.0 milliliters of a 1.5 M #HCl#?
1 Answer
Jun 12, 2016
62.5 mL of HCl is required.
Explanation:
For this type of problem, we want to use the following dilution formula:
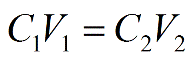
* TIP. * Whenever you are diluting a solution (going from a highly concentrated substance to a less concentrated substance by increasing the volume of the solvent) you always use this equation.
We know the initial concentration, final volume, and final concentration. All we have to do rearrange the equation to solve for the initial volume:

