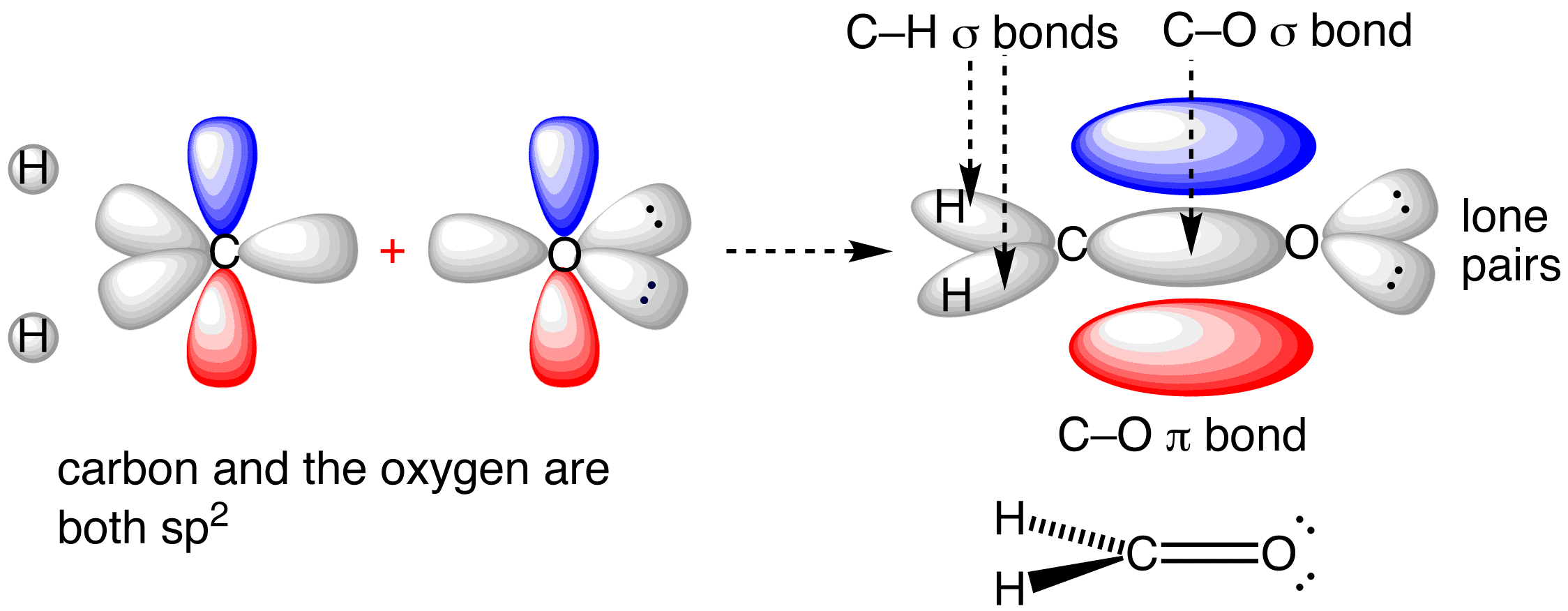
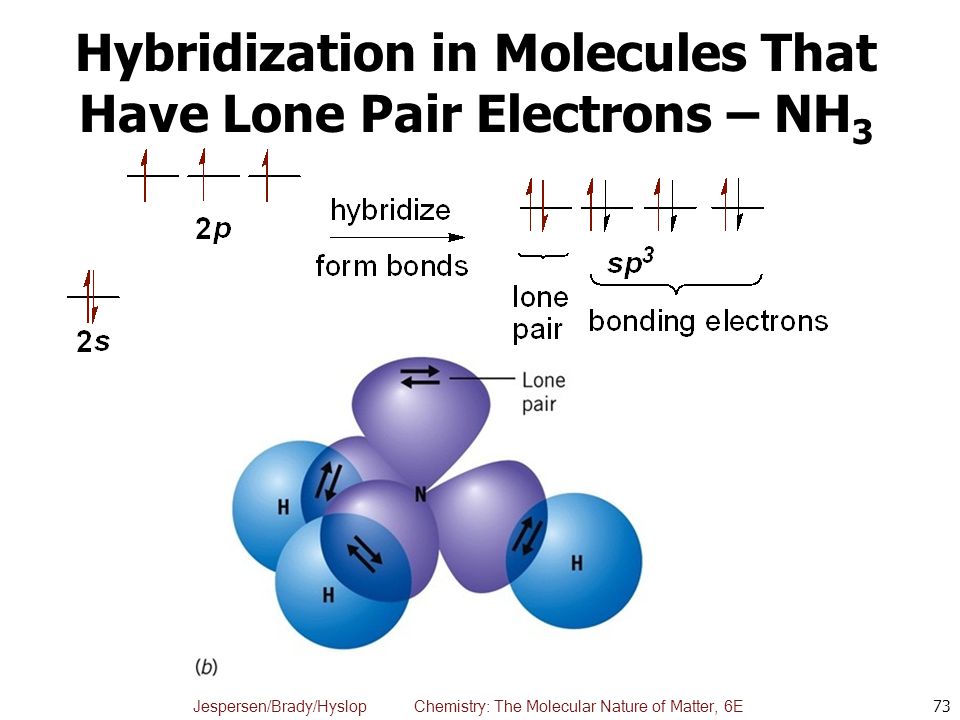
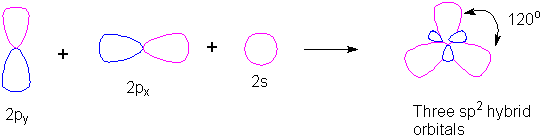How do you determine which orbitals are used in hybridization?
2 Answers
Yes. For general chemistry, we just count the number of electron groups around the central atom, and assume that the orbitals used are in order of angular momentum
"NORMAL", GENERAL CHEMISTRY WAY
We assume an ordering of
#s, p, p, p, d, d, d, d, d, f, f, f, f, f, f, f#
or
#sp^3d^5f^7# .
For example, in
(You can think of it as the central atom "anticipating" future bonds it can possibly make.)
Or, in
In short, count the orbitals from left to right in the list above until you have the same number of orbitals as the number of electron groups.
MORE RIGOROUS WAY
There is a more rigorous way to do it using Group Theory. You do not have to know this for general chemistry, but I am just providing it for the interested reader.
Here it is in a scratchpad:
https://socratic.org/scratchpad/25f1d95969583d1e1c9d
Here's a method using the VSEPR Theory to determine number of hybrid orbitals needed when using the Valence Bond Theory.
Explanation:
Here's a method that uses the VSEPR theory to determine how many hybrid orbitals would be needed for the central element of a given binary compound.
Need:
1. number of bonded electron pairs
2. number of non-bonded electron pairs
3. (NBPr + BPr)/2 => Parent Geometry => Number of Hybrids needed from central element's valence electrons.
Note: The detailing of this procedure will appear to be extensive, however, once the sequence is understood & with a bit of practice, these can almost be determined by inspection. I will finish with several examples of how this is a 'short cut' system.
DETAILED VERSION:
Example: Determine the geometry of the following:
(BPr) = Bonded Pr (BPr) = 2 => That is, 2 substrates attached to central element)
(NBPr) = NonBonded Pr (NBPr)
=
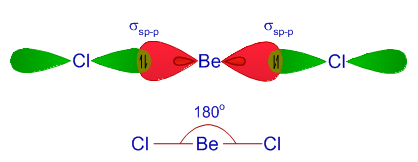
Valence Number = Number of Valence Electrons in given compound = Be + 2Cl = 1(2) + 2(7) = 16
Substrate Number = Number of Octet electrons associated with bonded substrates in given compound = 2Cl = 2(8) = 16 (Duet if working with Hydrogen as a substrate)
Total Electron Pairs Associated with Central Element of Parent Geometry = BPr + NBPr = 2 + 0 = 2 =>
The TTL
That is, 2 sp hybrid orbitals. sp hybrids => linear geometry
ABREVIATED VERSION:
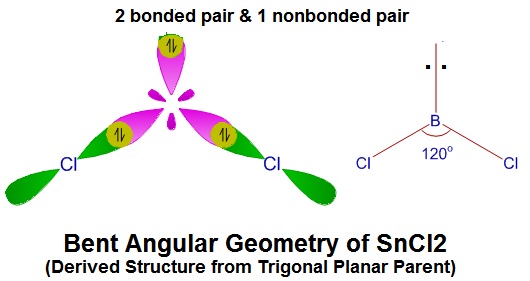
Determine geometry of
(Is this linear of a derived structure?)
#V = 1Sn + 2Cl = 1(4) + 2(7) = 18#
#S = 2Cl = 2(8) = 16#
Parent = BPr + NBPr = 2 + 1 = 3 => Trigonal Planar Parent
=> Derived Geometry
=> This is also the number of hybrid orbitals needed. =>
Determine the Geometry of
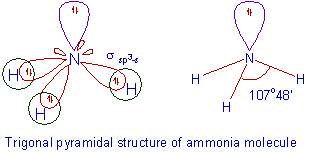
(Is this Trigonal Planar or a Derived Structure?)
#V = 1N + 3H = 1(5) + 3(1) = 8#
#S = 3H = 3(2) = 6#
BPr + NBPr = 3 + 1 = 4 => Tetrahedral Parent
Determine the Geometry of
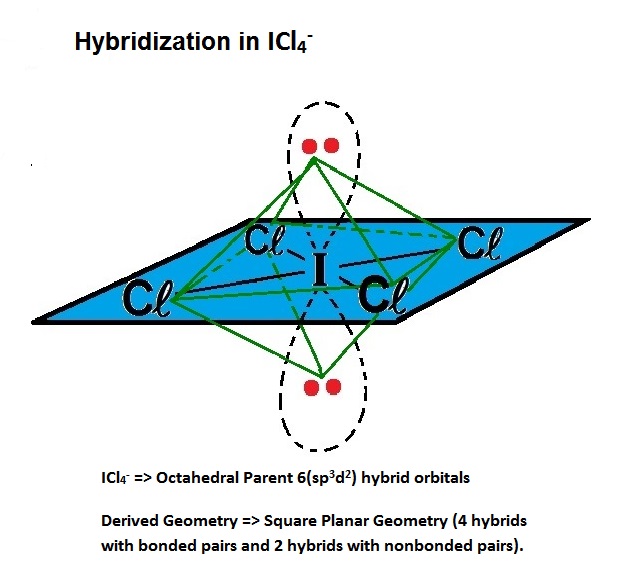
(Is this a Tetrahedral Geometry or a Derived Structure?)
#V = 1I + 4Cl + 1e^- = 1(7) + 4(7) + 1 = 36#
#S = 4Cl = 4(8) = 32#
BPr + NBPr = 4 + 2 = 6 => Octahedral Parent
STURCTURES WITH MULTIPLE BONDS
Determine the Geometry of
(The Lewis Structure shows this to be
BPr = 3
NBPr = 0
BPr + NBPr = 3 => Trigonal Planar Geometry about Carbon. This means that the structure is composed of