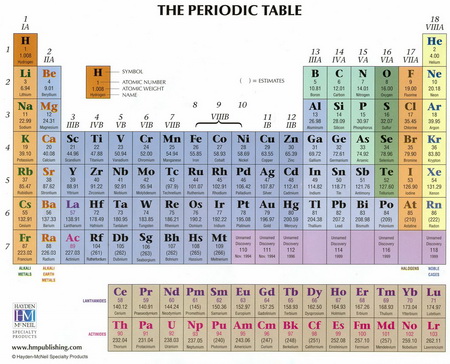
Why the entire d block starts with group IIIB and not IB (these are placed right after VIIIB)?
1 Answer
The numbers indicate approximately the highest oxidation number of the elements in each group.
Explanation:
The numbering is an old system called the CAS System (Chemical Abstracts Services System).
The main-group elements (Groups 1,2, and 13 to 18) were assigned to the A series, and the
The numbers indicated approximately the highest oxidation number of the elements in each group.
Thus, the assignments were:
Here's an example of a Periodic Table that uses both the CAS system and the new IUPAC numbering.
(From Alice in Galaxyland)

