How does ionic radius change if an atom gains electrons?
1 Answer
Would you not expect the ionic radius be LARGER than the atomic radius, should the atom undergo reduction........
Explanation:
And so you reduce an element, and typically the reduction reaction will occur with oxidizing reagents such as dioxygen and difluorine.....
We would reasonably expect that the anionic radius would be LARGER than the atomic radius given that we add an electron upon reduction, and this experiences shielding, electron-electron repulsion.
On the other hand, upon oxidation we remove an electron from the valence shell. And thus upon oxidation ionic radius should DECREASE upon oxidation. Agreed?
At any rate, as chemists, as physical scientists, we should interrogate the data.
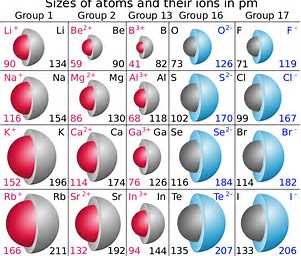
Do the given data, quoted in

