Is sodium chloride a mixture?
1 Answer
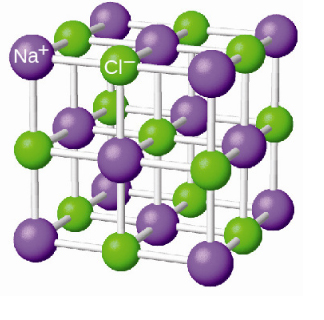
Sodium chloride solid is not a mixture. It is a pure substance... It cannot be physically separated into its components,
However, if for some reason, you meant
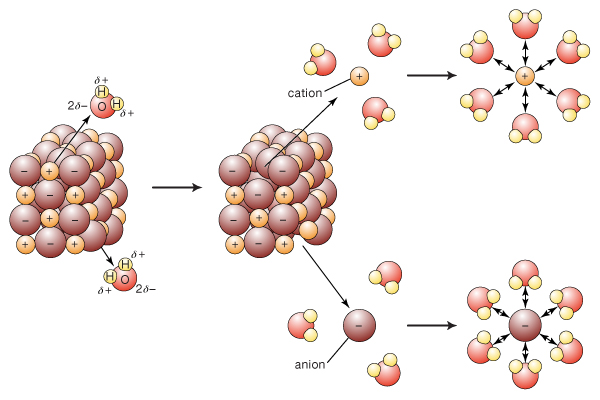
As a further note, the ions were held together by electrostatic attractions in a lattice, so no chemical change actually occurred!
And in water, this gives:
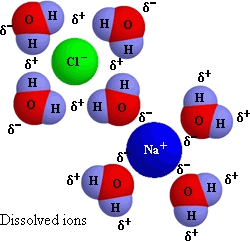
(This qualifies as a mixture, since evaporation allows reformation of sodium chloride solid again, thus separating the components,

