Question #d87eb
1 Answer
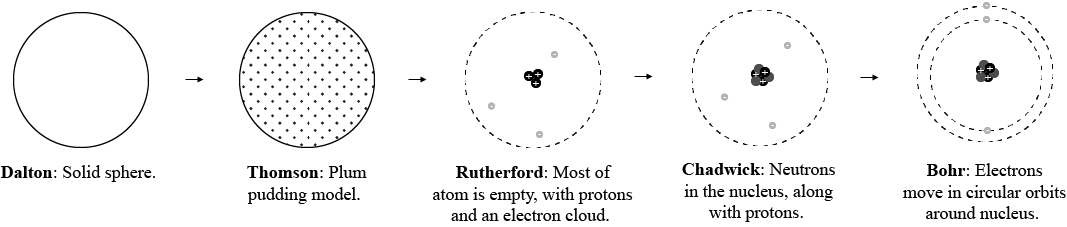
Explanation:
First, we have Dalton's model of the atom.
It was basically a solid sphere—no subatomic particles.

Then, J. J. Thomson conducted what is known as the cathode ray experiment.
He showed that atoms contained negatively charged subatomic particles called electrons.
From this, he created the plum pudding model of the atom:

Rutherford, through his gold foil experiment, found out that electrons were not actually interspersed within the atom—which was what J. J. Thomson had previously suggested.
He showed that atoms had a nucleus with positively charged protons. Electrons were moving outside of the nucleus in an electron cloud.

In 1932, Chadwick discovered the existence of a neutral particle inside the nucleus. It's called the neutron.
To account for this, the atomic model changed once again:

Then, Niels Bohr proposed that electrons, instead of being in an electron cloud, moved in orbits around the nucleus.

In more recent years, the work of scientists like Schrödinger and Born have shown that Bohr's model was incorrect.
An essential idea for this quantum mechanical model is that electrons do not orbit the nucleus like planets orbiting the sun.
Here are links to read, if you want more information on the quantum mechanical model! :)
(All images were made by me.)

