Are the following compounds enantiomer?
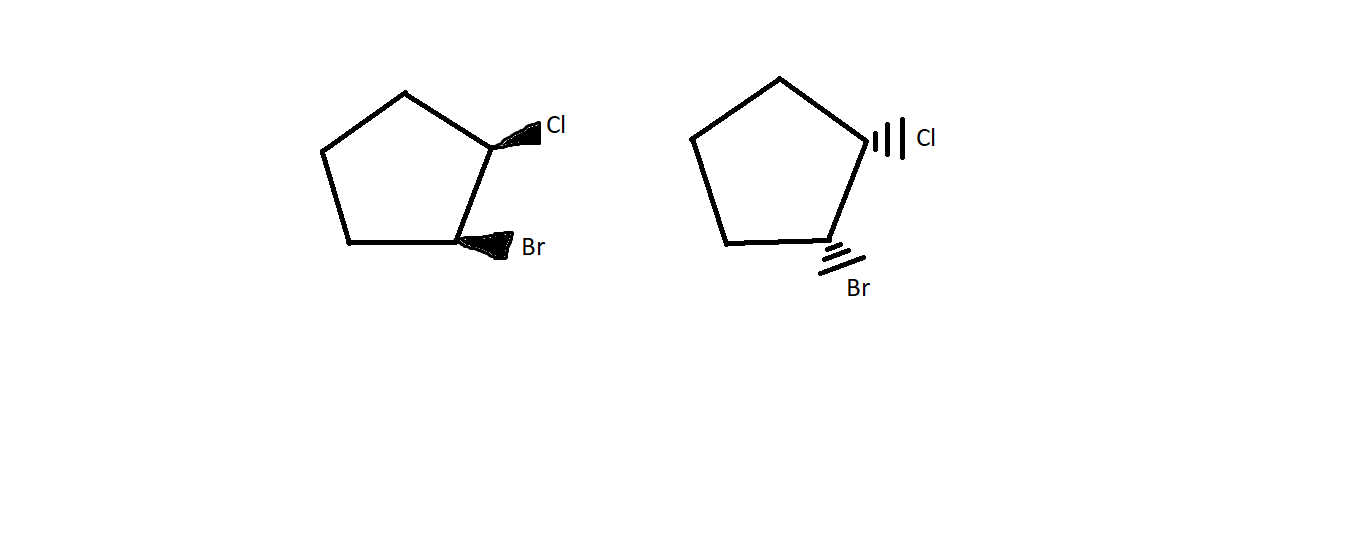
My book said "yes". Many people told me to just rotate the compound 180 degrees along the vertical axis. But that would only work if the compound is planar. But in this case, it is not. Can anyone help me?

My book said "yes". Many people told me to just rotate the compound 180 degrees along the vertical axis. But that would only work if the compound is planar. But in this case, it is not. Can anyone help me?
2 Answers
The structure on the left as the
Now, rotate the left-hand structure so that the
Yes, they are enantiomers.
Explanation:
One way to see that they are enantiomers is to slide the left-hand compound over the one on the right.
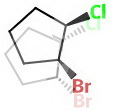
Then, the plane of the screen is the mirror.
The groups that are closest to your eye (the wedges) are furthest from your eye (dashes) in the mirror image.
Another way is to rotate the right-hand molecule 180° about the axis passing through
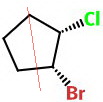
The bonds that were dashes on the right-hand side move to the left-hand side and become wedges.
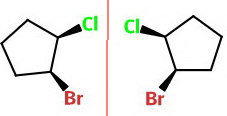
The mirror plane perpendicular to the plane of the screen shows that the two compounds are mirror images of each other.


