How can you carry out this conversion?
Describe giving by relevant equations how you would carry out the following conversions.
#"CrO"_4""^(2-) to "CrO"_7""^(2-)#
Describe giving by relevant equations how you would carry out the following conversions.
1 Answer
May 31, 2018
Add an acid to a solution of sodium chromate.
Explanation:
I am guessing that you meant to type
Chromate and dichromate ions are in equilibrium in aqueous soln.
#2underbrace(color(yellow)("CrO"_4^"2-"))_color(yellow)("yellow") + "2H"^"+" ⇌ underbrace(color(orange)("Cr"_2"O"_7^"2-"))_color(orange)("orange") + "H"_2"O"#
The position of equilibrium depends on the pH.
Per Le Châtelier's Principle, the addition of acid will push the position of equilibrium to the right.
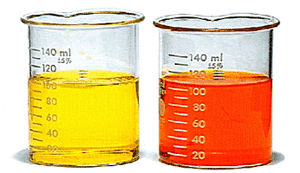
Thus, if we add sulfuric acid to a solution of potassium chromate, the yellow colour will change to orange.

