Calculate total number of optical isomers in the following compound CH3(CHOH)3CH3 ??
2 Answers
In this compound there are 4 optical isomers; this is calculated by multiplying the number of chiral centres by two.
Explanation:
The number of optical isomers in a compound is determined by the number of chiral centres in it. A chiral centre is a carbon atom that is bonded to four different molecules or atoms. Each chiral centre will result in two different optical isomers.
So, to work out the number of chiral centres, draw out the displayed formula of the compound, and circle/highlight any carbons in the compound that have four different molecules attached to them (to check this, try drawing the molecule as a tetrahedral shape around your chosen chiral centre; each bond from the central C atom should go to a unique compound). Then, just multiply the number of chiral centres by two to give the number of optical isomers.
Diagrams:
So below is your molecule (I have used MolView to sketch this):
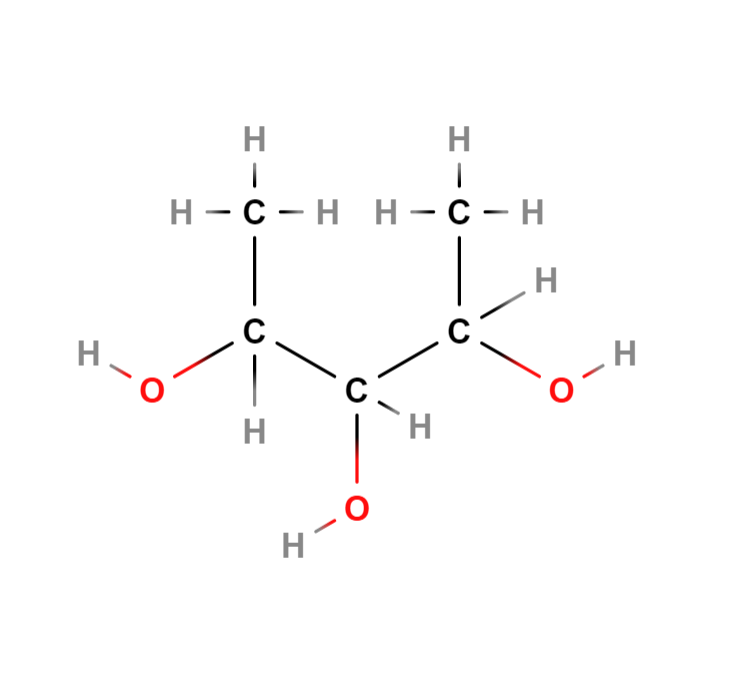
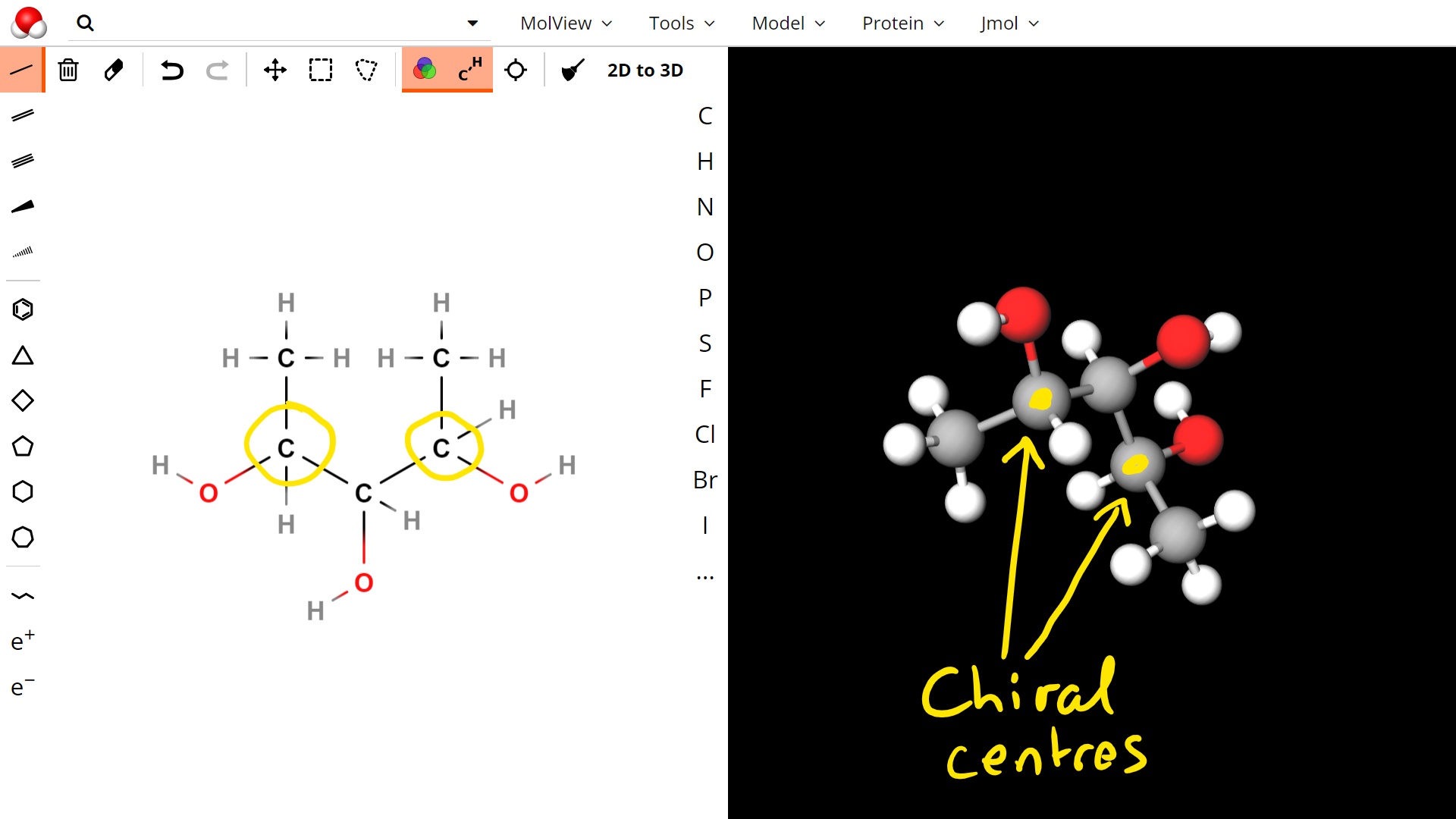
To work out which carbons are chiral, you just have to check each one separately. A chiral carbon is usually not on a branched group, or on the end of a chain, so often you can eliminate those pretty quickly. Below is the molecule with the chiral centres highlighted in yellow:
Theory of optical isomerism:
Stereoisomers are compounds that have the same structural formulae, but due to the lack of rotation around a double carbon bond (aka
Optical isomers (aka enantiomers) follow a similar principle, except instead of thinking about differences in location above and below a double bond, we are thinking about the orientation around a tetrahedral carbon centre.
The rule is that if each of the four substituents that are bonded to the central carbon are different, then it is possible for a stereoisomer of this compound to exist which is a mirror image of this compound. It is a form of stereoisomerism because both compounds have the same structural formulae, but cannot be superimposed on top of each other no matter how you rotate them.
The easiest way to get a feel for this is to draw a tetrahedral molecule, with (any) four different bonding groups; then imagine a mirror line next to the compound and draw its mirror image on the other side (this effect can be achieved by using a real mirror). Then, try and superimpose one molecule on top of the other - it should not be possible.
There are six optical isomers of
Explanation:
The maximum number of optical isomers is
Here,
Let's draw the eight possible structures as Fischer projections and count the unique isomers.
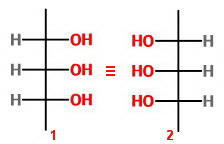
Isomers 1 and 2 represent the same molecule because of an internal plane of symmetry.
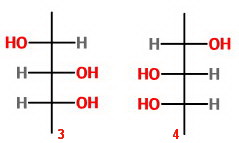
Isomers 3 and 4 are a pair of enantiomers.
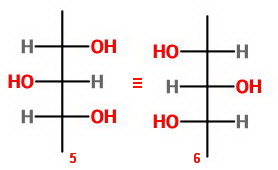
Isomers 5 and 6 represent the same molecule because of an internal plane of symmetry.
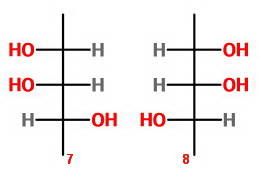
Isomers 7 and 8 are a pair of enantiomers.
Thus, there are six optical isomers of
But isn't the central carbon achiral?
No, it is a pseudoasymmetric carbon.
A pseudoasymmetric carbon is bonded to four different groups, two of which have the same constitution but opposite chirality.
If we assign configurations to isomers 1 and 4, we see that
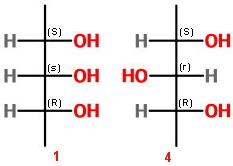
We designate the chirality of
Thus, Compound 1 is (
and Compound 2 is (


