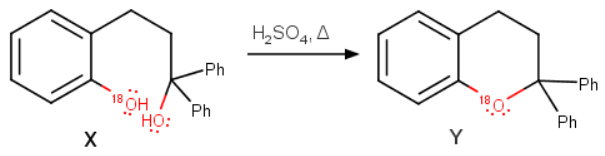
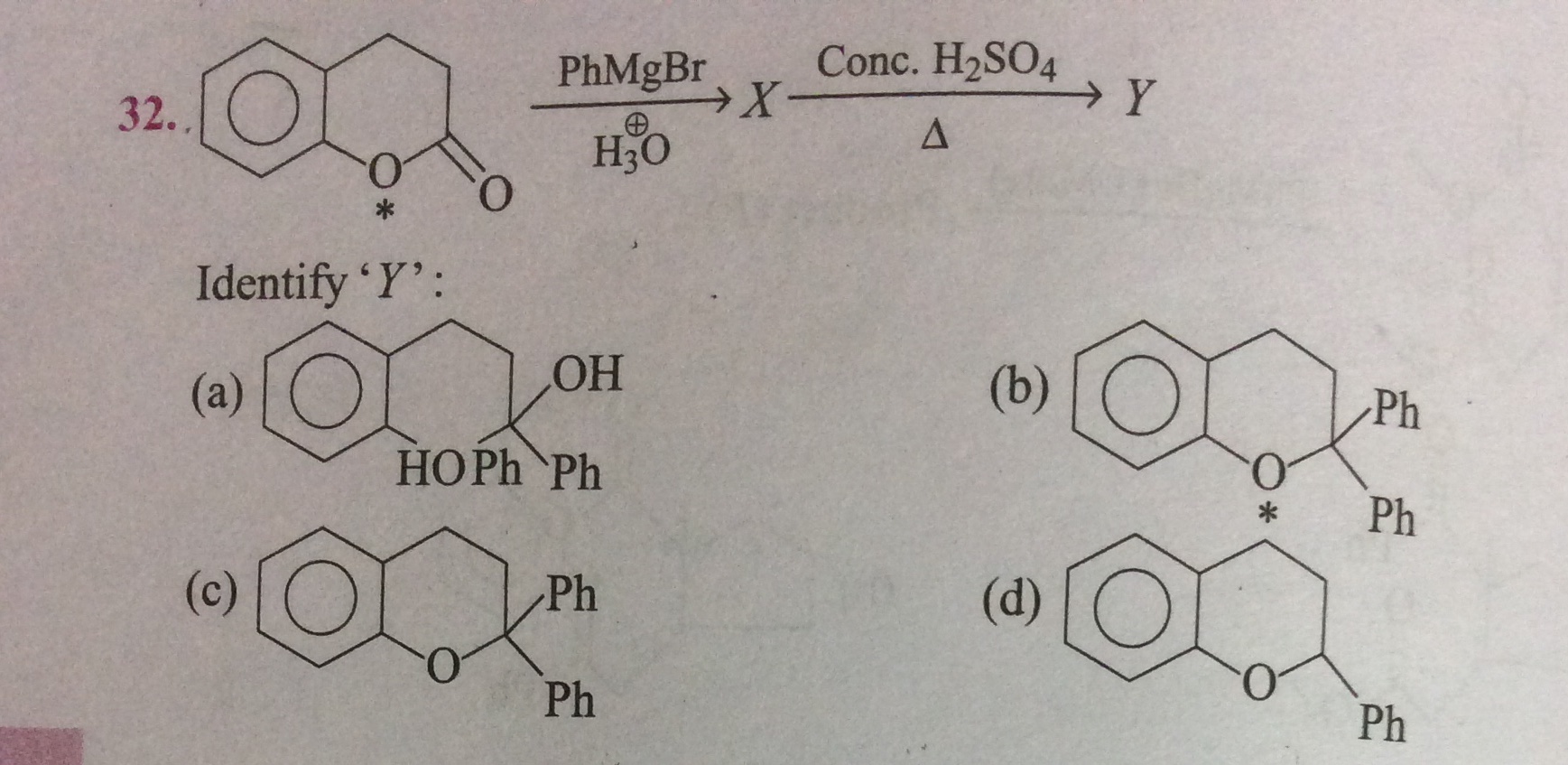
Identify ‘Y’?
1 Answer
The correct answer is (b).
Explanation:
Step 1. Grignard reaction
I m guessing that the starred
The first step in the formation of
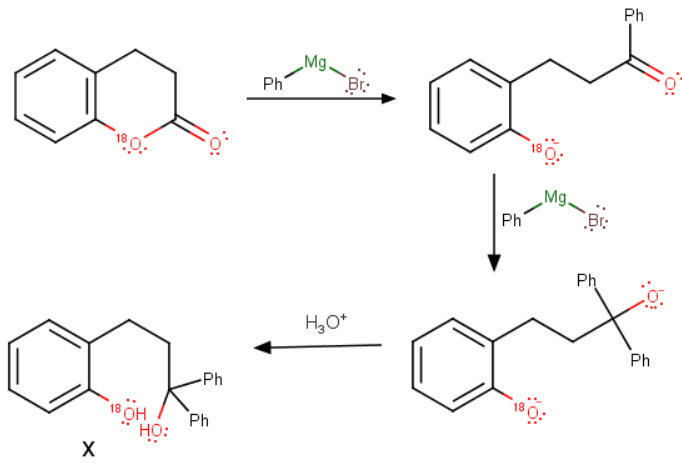
-
The Grignard reagent first converts the lactone to a phenyl ketone.
-
A second mole of the reagent then adds to the ketone to form a tertiary alcohol after acid workup.
-
The labelled oxygen remains on the original phenyl group.
Step 2. Acid-catalyzed formation of an ether
The formation of
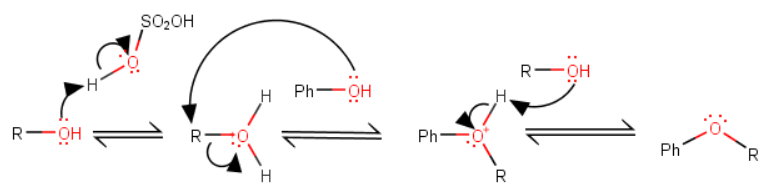
The steps in the mechanism are:
-
Sulfuric acid protonates the alcohol and converts it into a better leaving group.
-
The phenol displaces a water molecule from the water molecule and forms a protonated ether.
-
A molecule of excess alcohol deprotonates the ether
Again, note that the phenol oxygen stays attached to the ring.
Thus, we can represent the formation of