What charge would be needed on F2 to generate an ion with a bond order of 2?
1 Answer
You must generate a charge of +2.
Explanation:
Bond order in fluorine
Below is a molecular orbital diagram for a fluorine molecule.

The formula for bond order (BO) is
#color(blue)(bar(ul(|color(white)(a/a)"BO" = "B-A"/2color(white)(a/a)|)))" "#
where
How do we change the bond order?
We change the number of electrons, but do we add or remove them?
The highest occupied molecular orbitals (HOMOs) are antibonding, and so is the lowest unoccupied molecular orbital (LUMO).
If we add electrons, they will go into the antibonding LUMO and decrease the bond order.
We must remove electrons from the antibonding HOMOs.
Each electron contributes 0.5 to the bond order, so we remove one electron each from the
The energy level diagram then looks like this.
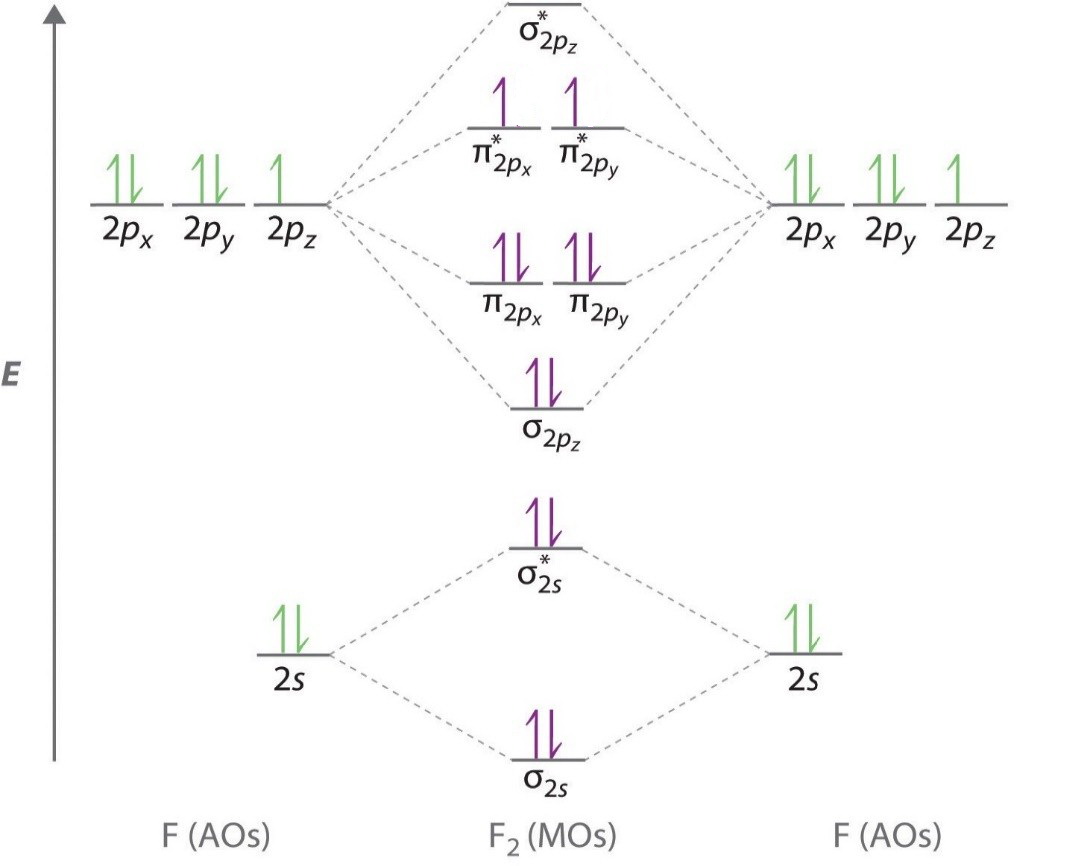
(Adqpted from eMedicalPrep)
We have removed two electrons, so the molecule becomes the ion

