Why teritary amine can't show chirality?
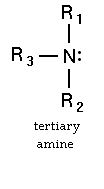
Why 3 degree amine is not chiral ?
Why 3 degree amine is not chiral ?
1 Answer
Tertiary amines are chiral, but they exist as racemic mixtures because of nitrogen inversion.
Explanation:
We know that a carbon atom with four different groups is chiral.
Well, the nitrogen atom in a 3° amine might also be chiral.
It has a trigonal pyramidal geometry, with a lone pair serving as the fourth "group."
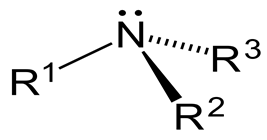
It is not superimposable on its mirror image, so it should be optically active, right?
Wrong!
Why aren't 3° amines optically active?
The answer lies in a phenomenon called amine inversion or nitrogen inversion.

The inversion occurs because the nitrogen atom can rehybridize to a planar
The result is an optically inactive racemic mixture of the two rapidly-interconverting enantiomeric forms.
The activation energy for inversion is low, so the inversion rate at room temperature for many 3° amines ranges from
Thus, separation of the enantiomers is impossible at room temperature.

