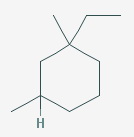
Can we name the compound below as 1-#sec#-butyl-4-methylcyclohexane?
1 Answer
Aug 31, 2014
No, we can't.
Explanation:
The structure of the molecule is

There are two reasons why you can't do this.
- A sec-butyl group has only one substituent on C-2 of the four-carbon chain. This structure has two groups attached, the CH₂ groups at C-2 and C-6 of the cyclohexane ring.
- You would be counting the same carbon atom twice. C-1 of the cyclohexane ring would be the same as C-2 of the butyl group.
You also see this compound named correctly as cis- and trans-1-ethyl-1,3-dimethylcyclohexane. This identifies the stereoisomers.
You may see it named incorrectly as (E)- and (Z)-1-ethyl-1,3-dimethylcyclohexane. The E and Z designations apply only to alkenes.

