How does a hydrogen atom in water form four hydrogen bonds?
1 Answer
No hydrogen atom is capable of forming hydrogen bonds with 4 other atoms, one hydrogen atom can form a hydrogen bond with only one other atom.
Explanation:
No hydrogen atom is capable of forming hydrogen bonds with 4 other atoms.
One hydrogen atom can form a hydrogen bond to only one other atom.
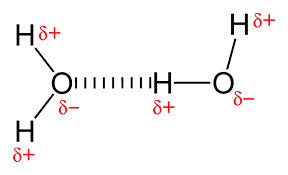
But a water molecule has two δ⁺ H atoms and two δ⁻ lone pairs.

Each H atom can donate a hydrogen bond to a lone pair on a nearby molecule.
And each lone pair can accept a hydrogen bond from a H atom on a nearby molecule.

So a single water molecule can form hydrogen bonds to four other molecules.

