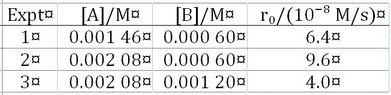
Given the data below, what are the orders of reaction with respect to #"[A]"# and #"[B]"#?
1 Answer
Mar 12, 2015
The orders are
Explanation:
A typical rate law is

From Expts. 2 and 1:
From Expts. 2 and 3:
Note: The results are not integers. I presume that this is just an exercise to demonstrate how to find orders of reaction by the method of initial rates.

