Question #db381
1 Answer
I think I get what you were thinking.
Normally, it is enthalpically unfavorable for iodine to perform an addition reaction with an alkene to get vicinal diiodides, and a big reason why is its large radius. Iodine generally makes pretty weak bonds anyways because of that, so it's more likely to simply exist as iodide with a carbocation in solution.
This can be shown by realizing that hydroiodic acid (
https://en.wikipedia.org/wiki/Bond_length
If you further note that carbon has a larger radius than hydrogen, it follows that the typical
And it's the weakest carbon-halogen bond of all the halogens.

So naturally, one might expect that heating the solution would contribute energy towards making a carbon-iodine bond, because more heat = more motion = faster reaction. But it really doesn't help make the bond. Why?
Because high temperatures promote elimination reactions (even though iodide is a weak base, a high enough heat promotes the kinetics of an elimination reaction). In other words, you're promoting both the formation and the elmimination of the iodine.
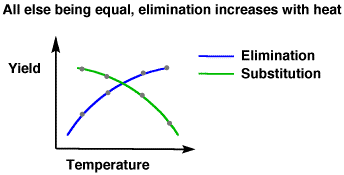
So, that even if iodine could stay bonded to carbon well, it could just as easily come back off via a
This can happen whenever the iodides are cis to each other (due to the rotation of the
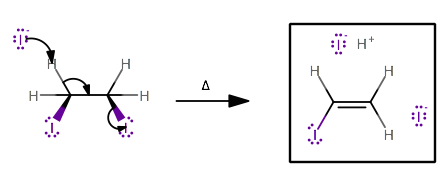
This could happen one more time, but either way, it isn't giving you an alkyl iodide.

