We are given the Fischer projection of 2-methylbutanoate.
#"C1"# is the carboxylate carbon, #"C2"# is at the centre of the cross, #"C3"# is the #"CH"_2# of the ethyl group, and #"C4"# is at the bottom. For argument's sake, let's call the horizontal methyl group #"C5"#.
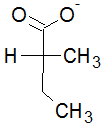
We must remember that, in a Fischer projection, the horizontal bonds are wedges and pointing towards us, while the vertical bonds are dashes and pointing behind the paper.
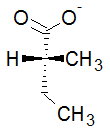
To get this into a perspective diagram, we rotate the molecule slightly to bring #"C1"# into the plane of the paper. #"C3"# moves further behind the paper.
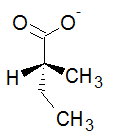
The perspective formula should have a wedge, a dash, and two bonds in the plane of the paper. The #"H"# should be on a wedge or a dash.
I'll rotate the #"C5"# backwards to put it in the plane of the paper. The #"H"# stays a wedge, and #"C3"# rotates slightly left as a dash.
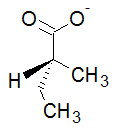
Now, we are ready to assign priorities.
The atoms directly attached to the chiral carbon (#"C2"#) are #"C1, C3, C5"#, and #"H"#.
#"H"# is obviously Priority 4, but the three #"C"# atoms are tied.
We must go to the atoms next further out to break the tie.
The atoms next further out from #"C1"# are (#"O,O,O"#) (since #"=O"# counts as two #"O"# atoms).
From #"C3"#, they are (#"C4,H,H"#).
From #"C5"#, they are (#"H,H,H"#).
The order of priority is #"O > C > H"#, so #"C1"# is Priority 1, #"C3"# is Priority 2, and #"C5"# is Priority 3.
If we count from #"C1 → C3 → C5"#, the circle goes counterclockwise (#S#).
However, the #"H"# is in front (not behind) so the configuration is #R#.
The structure is (#R#)-2-methylbutanoate.





