Well, we define #"aromaticity"# by specifying #(4n+2)pi# electrons that are constrained in a ring, and delocalized about that ring. Both benzene, #C_6H_6#, and cylcopentadienyl anion, #C_5H_5^-# meet these criteria. Because of the delocalization around the ring, these systems are especially stable.
And #"antiaromaticity"# specifies #(4n)pi# electrons that are constrained in a ring. This is an inherently unstable system, and a species such as #"cyclo-butadiene"#, #C_4H_4#, is a transient short-lived intermediate, whose existence requires special experiments to verify.
Cyclooctatetraene, #C_8H_8# is a real species that would be antiaromatic BUT for the fact that the tetraene buckles in solution to form a barrel (and clearly the buckling is due to the thermodynamic instability of the planar conformer):
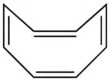
On the other hand, #"1,3,5,7-cyclooctatetraene"#, may be reduced fairly easily with potassium metal.........
#C_8H_8+2Krarr[eta^(8)-C_8H_8]^(2-)K_2^(+)#
.........to give the PLANAR #C_8H_8^(2-)# dianion, which does fulfil the Huckel criterion (#10pi# electrons), and #"COT dianion"# has an extensive coordination chemistry, with uranocene, #[U(eta^8-C_8H_8)_2]# isolated as an excellent example of a large sandwich complex.

